The plasminogen activation system reduces fibrosis in the lung by a hepatocyte growth factor-dependent mechanism
- PMID: 14982862
- PMCID: PMC1614722
- DOI: 10.1016/S0002-9440(10)63196-3
The plasminogen activation system reduces fibrosis in the lung by a hepatocyte growth factor-dependent mechanism
Abstract
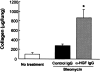
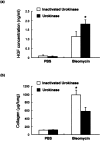
Mice deficient in the plasminogen activator inhibitor-1 gene (PAI-1-/- mice) are relatively protected from developing pulmonary fibrosis from bleomycin administration. We hypothesized that one of the protective mechanisms may be the ability of the plasminogen system to enhance hepatocyte growth factor (HGF) effects, which have been reported to be anti-fibrotic in the lung. HGF is known to be sequestered in tissues by binding to extracellular matrix components. Following bleomycin administration, we found that HGF protein levels were higher in bronchoalveolar lavage fluid from PAI-1-/- mice compared to wild-type (PAI-1+/+) mice. This increase could be suppressed by administering tranexamic acid, which inhibits plasmin activity. Conversely, intratracheal instillation of urokinase into bleomycin-injured PAI-1+/+ mice to activate plasminogen caused a significant increase in HGF within bronchoalveolar lavage and caused less collagen accumulation in the lungs. Administration of an anti-HGF neutralizing antibody markedly increased collagen accumulation in the lungs of bleomycin-injured PAI-1-/- mice. These results support the hypothesis that increasing the availability of HGF, possibly by enhancing its release from extracellular matrix by a plasmin-dependent mechanism, is an important means by which activation of the plasminogen system can limit pulmonary fibrosis.
Figures
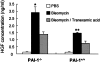

Similar articles
-
Depressing time: Waiting, melancholia, and the psychoanalytic practice of care.In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. PMID: 36137063 Free Books & Documents. Review.
-
Highly Calibrated Relationship Between Bleomycin Concentrations and Facets of the Active Phase Fibrosis in Classical Mouse Bleomycin Model.Int J Mol Sci. 2024 Nov 15;25(22):12300. doi: 10.3390/ijms252212300. Int J Mol Sci. 2024. PMID: 39596365 Free PMC article.
-
Qualitative evidence synthesis informing our understanding of people's perceptions and experiences of targeted digital communication.Cochrane Database Syst Rev. 2019 Oct 23;10(10):ED000141. doi: 10.1002/14651858.ED000141. Cochrane Database Syst Rev. 2019. PMID: 31643081 Free PMC article.
-
Ceftazidime with avibactam for treating severe aerobic Gram-negative bacterial infections: technology evaluation to inform a novel subscription-style payment model.Health Technol Assess. 2024 Oct;28(73):1-230. doi: 10.3310/YAPL9347. Health Technol Assess. 2024. PMID: 39487661 Free PMC article.
-
Valley fever under a changing climate in the United States.Environ Int. 2024 Nov;193:109066. doi: 10.1016/j.envint.2024.109066. Epub 2024 Oct 11. Environ Int. 2024. PMID: 39432997 Review.
Cited by
-
Libby amphibole-induced mesothelial cell autoantibodies bind to surface plasminogen and alter collagen matrix remodeling.Physiol Rep. 2016 Aug;4(15):e12881. doi: 10.14814/phy2.12881. Physiol Rep. 2016. PMID: 27519611 Free PMC article.
-
Chemical pleurodesis - a review of mechanisms involved in pleural space obliteration.Respir Res. 2019 Nov 7;20(1):247. doi: 10.1186/s12931-019-1204-x. Respir Res. 2019. PMID: 31699094 Free PMC article. Review.
-
Urokinase-type plasminogen activator increases hepatocyte growth factor activity required for skeletal muscle regeneration.Blood. 2009 Dec 3;114(24):5052-61. doi: 10.1182/blood-2008-12-196212. Epub 2009 Oct 7. Blood. 2009. PMID: 19812386 Free PMC article.
-
Mannose receptor 2 attenuates renal fibrosis.J Am Soc Nephrol. 2012 Feb;23(2):236-51. doi: 10.1681/ASN.2011030310. Epub 2011 Nov 17. J Am Soc Nephrol. 2012. PMID: 22095946 Free PMC article.
-
Mechanosignaling through YAP and TAZ drives fibroblast activation and fibrosis.Am J Physiol Lung Cell Mol Physiol. 2015 Feb 15;308(4):L344-57. doi: 10.1152/ajplung.00300.2014. Epub 2014 Dec 12. Am J Physiol Lung Cell Mol Physiol. 2015. PMID: 25502501 Free PMC article. Clinical Trial.
References
-
- Kuhn C, 3rd, Boldt J, King TE, Jr, Crouch E, Vartio T, McDonald JA. An immunohistochemical study of architectural remodeling and connective tissue synthesis in pulmonary fibrosis. Am Rev Respir Dis. 1989;140:1693–1703. - PubMed
-
- Kuhn C. The pathogenesis of pulmonary fibrosis. Monogr Pathol. 1993:78–92. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

