Carbachol regulation of rabbit ileal brush border Na+-H+ exchanger 3 (NHE3) occurs through changes in NHE3 trafficking and complex formation and is Src dependent
- PMID: 14978207
- PMCID: PMC1664999
- DOI: 10.1113/jphysiol.2004.060921
Carbachol regulation of rabbit ileal brush border Na+-H+ exchanger 3 (NHE3) occurs through changes in NHE3 trafficking and complex formation and is Src dependent
Abstract
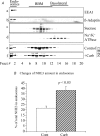
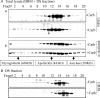
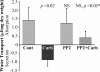
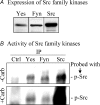
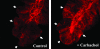
The epithelial brush border membrane (BBM) Na(+)-H(+) exchanger 3 (NHE3) is the major transport protein responsible for ileal electroneutral Na(+) absorption. We have previously shown that ileal BBM NHE3 activity is rapidly inhibited by carbachol, an agonist that mimics cholinergic activation in digestion. In this study, we investigated the mechanisms involved in this NHE3 inhibition. Carbachol decreased the amount of ileal Na(+) absorptive cell BBM NHE3 within 10 min of exposure. Based on OptiPrep gradient centrifugation, carbachol increased the amount of NHE3 in early endosomes and decreased the amount of NHE3 in BBM, consistent with effects on NHE3 trafficking. The decrease in BBM NHE3 occurred in the detergent-soluble BBM fraction with no change in the amount of NHE3 in the BBM detergent-resistant membranes. The size of BBM NHE3 complexes increased in carbachol-exposed ileum, as studied with sucrose gradient centrifugation. The NHE3 complex size increased in the total BBM, but did not change in the detergent-soluble fraction. This suggests that carbachol treatment enhanced the association of proteins with NHE3 complexes specifically in the detergent-resistant fraction of ileal BBM. NHERF2, alpha-actinin-4 and protein kinase C were among those NHE3-associated proteins because they were more efficiently coimmunoprecipitated from total BBM after carbachol treatment. Moreover, Src was involved in the carbachol-mediated inhibition since: (1) c-Src was rapidly activated in the detergent-resistant membranes by carbachol; and (2) carbachol inhibition of ileal Na(+) absorption was completely abolished by the Src family inhibitor 4-amino-5-(4-chlorophenyl)-7-(t-butyl)pyrazolo[3,4-d]pyrimidine (PP2). Moreover, the carbachol-induced increase in the size of NHE3-containing complexes was reversed by PP2. These data demonstrate that regulation of NHE3 activity by carbachol can be achieved at several interrelated levels: (1) the subcellular level, at which NHE3 is rapidly endocytosed from BBM to endocytic vesicles upon treatment with carbachol; (2) multiple BBM pools, in which carbachol selectively decreases the amount of NHE3 in the BBM detergent-soluble fraction but not the detergent-resistant membrane; and (3) the molecular level, at which NHE3 complex-associated proteins can be changed upon carbachol treatment, with carbachol leading to larger BBM NHE3 complexes and increased co-IP of NHERF2 with alpha-actinin-4 and activated PKC. The study further describes NHE3 presence simultaneously in multiple dynamic BBM pools in which NHE3 distribution and associated proteins are altered as part of carbachol-induced and Src-mediated rapid signal transduction, which decreases the amount of BBM NHE3 and thus inhibits NHE3 activity.
Figures



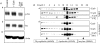
Similar articles
-
Akt2, phosphatidylinositol 3-kinase, and PTEN are in lipid rafts of intestinal cells: role in absorption and differentiation.Gastroenterology. 2004 Jan;126(1):122-35. doi: 10.1053/j.gastro.2003.10.061. Gastroenterology. 2004. PMID: 14699494
-
Asymmetric signal transduction in polarized ileal Na(+)-absorbing cells: carbachol activates brush-border but not basolateral-membrane PIP2-PLC and translocates PLC-gamma 1 only to the brush border.Biochem J. 1996 Jan 15;313 ( Pt 2)(Pt 2):509-18. doi: 10.1042/bj3130509. Biochem J. 1996. PMID: 8573085 Free PMC article.
-
Na+-H+ exchanger 3 (NHE3) is present in lipid rafts in the rabbit ileal brush border: a role for rafts in trafficking and rapid stimulation of NHE3.J Physiol. 2001 Dec 1;537(Pt 2):537-52. doi: 10.1111/j.1469-7793.2001.00537.x. J Physiol. 2001. PMID: 11731584 Free PMC article.
-
The epithelial brush border Na+/H+ exchanger NHE3 associates with the actin cytoskeleton by binding to ezrin directly and via PDZ domain-containing Na+/H+ exchanger regulatory factor (NHERF) proteins.Clin Exp Pharmacol Physiol. 2008 Aug;35(8):863-71. doi: 10.1111/j.1440-1681.2008.04931.x. Epub 2008 Apr 21. Clin Exp Pharmacol Physiol. 2008. PMID: 18430067 Review.
-
NHERF family and NHE3 regulation.J Physiol. 2005 Aug 15;567(Pt 1):3-11. doi: 10.1113/jphysiol.2005.090399. Epub 2005 May 19. J Physiol. 2005. PMID: 15905209 Free PMC article. Review.
Cited by
-
Scaffold protein connector enhancer of kinase suppressor of Ras isoform 3 (CNK3) coordinates assembly of a multiprotein epithelial sodium channel (ENaC)-regulatory complex.J Biol Chem. 2012 Sep 21;287(39):33014-25. doi: 10.1074/jbc.M112.389148. Epub 2012 Jul 31. J Biol Chem. 2012. PMID: 22851176 Free PMC article.
-
Regulation of the intestinal Na+/H+ exchanger NHE3 by AMP-activated kinase is dependent on phosphorylation of NHE3 at S555 and S563.Am J Physiol Cell Physiol. 2024 Jan 1;326(1):C50-C59. doi: 10.1152/ajpcell.00540.2023. Epub 2023 Dec 4. Am J Physiol Cell Physiol. 2024. PMID: 38047302 Free PMC article.
-
Elevated intracellular calcium stimulates NHE3 activity by an IKEPP (NHERF4) dependent mechanism.Cell Physiol Biochem. 2008;22(5-6):693-704. doi: 10.1159/000185553. Epub 2008 Dec 9. Cell Physiol Biochem. 2008. PMID: 19088451 Free PMC article.
-
Regulation of intestinal electroneutral sodium absorption and the brush border Na+/H+ exchanger by intracellular calcium.Ann N Y Acad Sci. 2009 May;1165:240-8. doi: 10.1111/j.1749-6632.2009.04055.x. Ann N Y Acad Sci. 2009. PMID: 19538312 Free PMC article. Review.
-
Elevated calcium acutely regulates dynamic interactions of NHERF2 and NHE3 proteins in opossum kidney (OK) cell microvilli.J Biol Chem. 2011 Oct 7;286(40):34486-96. doi: 10.1074/jbc.M111.230219. Epub 2011 Jul 28. J Biol Chem. 2011. PMID: 21799002 Free PMC article.
References
-
- Akhter S, Cavet ME, Tse CM, Donowitz M. C-terminal domains of Na+/H+ exchanger isoform 3 are involved in the basal and serum-stimulated membrane trafficking of the exchanger. Biochemistry. 2000;39:1990–2000. - PubMed
-
- Akhter S, Kovbasnjuk O, Li X, Cavet M, Noel J, Arpin M, Hubbard AL, Donowitz M. Na+/H+ exchanger 3 is in large complexes in the center of the apical surface of proximal tubule-derived OK cells. Am J Physiol. 2002;283:C927–C940. - PubMed
-
- Barrett KE. Positive and negative regulation of chloride secretion in T84 cells. Am J Physiol. 1993;265:C859–C868. - PubMed
-
- Biemesderfer D, Pizzonia J, Abu-Alfa A, Exner M, Reilly R, Igarashi P, Aronson PS. NHE3: a Na+/H+ exchanger isoform of renal brush border. Am J Physiol. 1993;265:F736–F742. - PubMed
-
- Chow JY, Carlstrom K, Barrett KE. Growth hormone reduces chloride secretion in human colonic epithelial cells via EGF receptor and extracellular regulated kinase. Gastroenterology. 2003;125:1114–1124. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

