Integration of TGF-beta/Smad and Jagged1/Notch signalling in epithelial-to-mesenchymal transition
- PMID: 14976548
- PMCID: PMC380966
- DOI: 10.1038/sj.emboj.7600069
Integration of TGF-beta/Smad and Jagged1/Notch signalling in epithelial-to-mesenchymal transition
Abstract
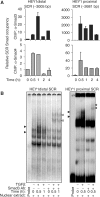
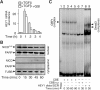
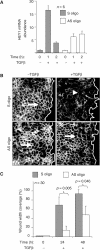
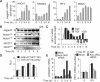
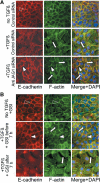
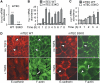
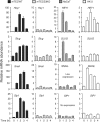
Epithelial-to-mesenchymal transitions (EMTs) underlie cell plasticity required in embryonic development and frequently observed in advanced carcinogenesis. Transforming growth factor-beta (TGF-beta) induces EMT phenotypes in epithelial cells in vitro and has been associated with EMT in vivo. Here we report that expression of the hairy/enhancer-of-split-related transcriptional repressor Hey1, and the Notch-ligand Jagged1 (Jag1), was induced by TGF-beta at the onset of EMT in epithelial cells from mammary gland, kidney tubules, and epidermis. The HEY1 expression profile was biphasic, consisting of immediate-early Smad3-dependent, Jagged1/Notch-independent activation, followed by delayed, indirect Jagged1/Notch-dependent activation. TGF-beta-induced EMT was blocked by RNA silencing of HEY1 or JAG1, and by chemical inactivation of Notch. The EMT phenotype, biphasic activation of Hey1, and delayed expression of Jag1 were induced by TGF-beta in wild-type, but not in Smad3-deficient, primary mouse kidney tubular epithelial cells. Our findings identify a new mechanism for functional integration of Jagged1/Notch signalling and coordinated activation of the Hey1 transcriptional repressor controlled by TGF-beta/Smad3, and demonstrate functional roles for Smad3, Hey1, and Jagged1/Notch in mediating TGF-beta-induced EMT.
Figures

Similar articles
-
BMP-7 counteracts TGF-beta1-induced epithelial-to-mesenchymal transition and reverses chronic renal injury.Nat Med. 2003 Jul;9(7):964-8. doi: 10.1038/nm888. Nat Med. 2003. PMID: 12808448
-
Targeted disruption of TGF-beta1/Smad3 signaling protects against renal tubulointerstitial fibrosis induced by unilateral ureteral obstruction.J Clin Invest. 2003 Nov;112(10):1486-94. doi: 10.1172/JCI19270. J Clin Invest. 2003. PMID: 14617750 Free PMC article.
-
A role for Id in the regulation of TGF-beta-induced epithelial-mesenchymal transdifferentiation.Cell Death Differ. 2004 Oct;11(10):1092-101. doi: 10.1038/sj.cdd.4401467. Cell Death Differ. 2004. PMID: 15181457
-
Chromatin structure regulation in transforming growth factor-beta-directed epithelial-mesenchymal transition.Cells Tissues Organs. 2007;185(1-3):162-74. doi: 10.1159/000101317. Cells Tissues Organs. 2007. PMID: 17587822 Review.
-
TGF-beta and epithelial-to-mesenchymal transitions.Oncogene. 2005 Aug 29;24(37):5764-74. doi: 10.1038/sj.onc.1208927. Oncogene. 2005. PMID: 16123809 Review.
Cited by
-
Beauvericin Reverses Epithelial-to-Mesenchymal Transition in Triple-Negative Breast Cancer Cells through Regulation of Notch Signaling and Autophagy.ACS Pharmacol Transl Sci. 2024 Sep 3;7(9):2878-2893. doi: 10.1021/acsptsci.4c00370. eCollection 2024 Sep 13. ACS Pharmacol Transl Sci. 2024. PMID: 39296261
-
MED30 Regulates the Proliferation and Motility of Gastric Cancer Cells.PLoS One. 2015 Jun 25;10(6):e0130826. doi: 10.1371/journal.pone.0130826. eCollection 2015. PLoS One. 2015. PMID: 26110885 Free PMC article.
-
Hairy/enhancer-of-split related with YRPW motif protein 1 promotes osteosarcoma metastasis via matrix metallopeptidase 9 expression.Br J Cancer. 2015 Mar 31;112(7):1232-40. doi: 10.1038/bjc.2015.84. Br J Cancer. 2015. PMID: 25742474 Free PMC article.
-
Aberrant Notch1-dependent effects on glomerular parietal epithelial cells promotes collapsing focal segmental glomerulosclerosis with progressive podocyte loss.Kidney Int. 2013 Jun;83(6):1065-75. doi: 10.1038/ki.2013.48. Epub 2013 Feb 27. Kidney Int. 2013. PMID: 23447065 Free PMC article.
-
Targeting the Epithelial-to-Mesenchymal Transition in Cancer Stem Cells for a Better Clinical Outcome of Glioma.Technol Cancer Res Treat. 2020 Jan-Dec;19:1533033820948053. doi: 10.1177/1533033820948053. Technol Cancer Res Treat. 2020. PMID: 33089751 Free PMC article. Review.
References
-
- Bakin AV, Rinehart C, Tomlinson AK, Arteaga CL (2002) p38 mitogen-activated protein kinase is required for TGFbeta-mediated fibroblastic transdifferentiation and cell migration. J Cell Sci 115: 3193–3206 - PubMed
-
- Bakin AV, Tomlinson AK, Bhowmick NA, Moses HL, Arteaga CL (2000) Phosphatidylinositol-3 kinase function is required for TGFbeta-mediated epithelial to mesenchymal transition and cell migration. J Biol Chem 275: 36803–36810 - PubMed
-
- Camenisch TD, Molin DG, Person A, Runyan RB, Gittenberger-De Groot AC, Mcdonald JA, Klewer SE (2002) Temporal and distinct TGFbeta ligand requirements during mouse and avian endocardial cushion morphogenesis. Dev Biol 248: 170–181 - PubMed
-
- Cano A, Perez-Moreno MA, Rodrigo I, Locascio A, Blanco MJ, Del Barrio MG, Portillo F, Nieto MA (2000) The transcription factor snail controls epithelial-mesenchymal transitions by repressing E-cadherin expression. Nat Cell Biol 2: 76–83 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

