A small molecular activator of cardiac hypertrophy uncovered in a chemical screen for modifiers of the calcineurin signaling pathway
- PMID: 14976250
- PMCID: PMC365712
- DOI: 10.1073/pnas.0308723101
A small molecular activator of cardiac hypertrophy uncovered in a chemical screen for modifiers of the calcineurin signaling pathway
Abstract
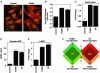
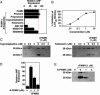
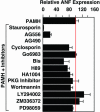
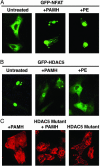
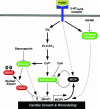
The calcium, calmodulin-dependent phosphatase calcineurin, regulates growth and gene expression of striated muscles. The activity of calcineurin is modulated by a family of cofactors, referred to as modulatory calcineurin-interacting proteins (MCIPs). In the heart, the MCIP1 gene is activated by calcineurin and has been proposed to fulfill a negative feedback loop that restrains potentially pathological calcineurin signaling, which would otherwise lead to abnormal cardiac growth. In a high-throughput screen for small molecules capable of regulating MCIP1 expression in muscle cells, we identified a unique 4-aminopyridine derivative exhibiting an embedded partial structural motif of serotonin (5-hydroxytryptamine, 5-HT). This molecule, referred to as pyridine activator of myocyte hypertrophy, acts as a selective agonist for 5-HT(2A/2B) receptors and induces hypertrophy of cardiac muscle cells through a signaling pathway involving calcineurin and a kinase-dependent mechanism that inactivates class II histone deacetylases, which act as repressors of cardiac growth. These findings identify MCIP1 as a downstream target of 5-HT(2A/2B) receptor signaling in cardiac muscle cells and suggest possible uses for 5-HT(2A/2B) agonists and antagonists as modulators of cardiac growth and gene expression.
Figures

Similar articles
-
Enabling Systemic Identification and Functionality Profiling for Cdc42 Homeostatic Modulators.bioRxiv [Preprint]. 2024 Jan 8:2024.01.05.574351. doi: 10.1101/2024.01.05.574351. bioRxiv. 2024. Update in: Commun Chem. 2024 Nov 19;7(1):271. doi: 10.1038/s42004-024-01352-7. PMID: 38260445 Free PMC article. Updated. Preprint.
-
Depressing time: Waiting, melancholia, and the psychoanalytic practice of care.In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. PMID: 36137063 Free Books & Documents. Review.
-
Signalosome-Regulated Serum Response Factor Phosphorylation Determining Myocyte Growth in Width Versus Length as a Therapeutic Target for Heart Failure.Circulation. 2020 Dec;142(22):2138-2154. doi: 10.1161/CIRCULATIONAHA.119.044805. Epub 2020 Sep 16. Circulation. 2020. PMID: 32933333 Free PMC article.
-
The Emerging Roles of Multimolecular G-Quadruplexes in Transcriptional Regulation and Chromatin Organization.Acc Chem Res. 2024 Dec 3;57(23):3397-3406. doi: 10.1021/acs.accounts.4c00574. Epub 2024 Nov 18. Acc Chem Res. 2024. PMID: 39555660 Free PMC article.
-
Trends in Surgical and Nonsurgical Aesthetic Procedures: A 14-Year Analysis of the International Society of Aesthetic Plastic Surgery-ISAPS.Aesthetic Plast Surg. 2024 Oct;48(20):4217-4227. doi: 10.1007/s00266-024-04260-2. Epub 2024 Aug 5. Aesthetic Plast Surg. 2024. PMID: 39103642 Review.
Cited by
-
Epigenetic regulation of the electrophysiological phenotype of human embryonic stem cell-derived ventricular cardiomyocytes: insights for driven maturation and hypertrophic growth.Stem Cells Dev. 2013 Oct 1;22(19):2678-90. doi: 10.1089/scd.2013.0125. Epub 2013 Jun 14. Stem Cells Dev. 2013. PMID: 23656529 Free PMC article.
-
Divergent signaling pathways mediate induction of Na,K-ATPase alpha1 and beta1 subunit gene transcription by low potassium.Mol Cell Biochem. 2007 Jan;294(1-2):73-85. doi: 10.1007/s11010-006-9247-y. Epub 2006 Aug 15. Mol Cell Biochem. 2007. PMID: 16909306
-
Differential metal content and gene expression in rat left ventricular hypertrophy due to hypertension and hyperactivity.J Trace Elem Med Biol. 2014 Jul;28(3):311-6. doi: 10.1016/j.jtemb.2014.02.002. Epub 2014 Feb 22. J Trace Elem Med Biol. 2014. PMID: 24629670 Free PMC article.
-
International Union of Basic and Clinical Pharmacology. CX. Classification of Receptors for 5-hydroxytryptamine; Pharmacology and Function.Pharmacol Rev. 2021 Jan;73(1):310-520. doi: 10.1124/pr.118.015552. Pharmacol Rev. 2021. PMID: 33370241 Free PMC article. Review.
-
STIM1-dependent store-operated Ca²⁺ entry is required for pathological cardiac hypertrophy.J Mol Cell Cardiol. 2012 Jan;52(1):136-47. doi: 10.1016/j.yjmcc.2011.11.003. Epub 2011 Nov 13. J Mol Cell Cardiol. 2012. PMID: 22108056 Free PMC article.
References
-
- Frey, N. & Olson, E. N. (2003) Annu. Rev. Physiol. 65, 45-79. - PubMed
-
- MacLellan, W. R. & Schneider, M. D. (2000) Annu. Rev. Physiol. 62, 289-319. - PubMed
-
- Olson, E. N. & Williams, R. S. (2000) Cell 101, 689-692. - PubMed
-
- Kannel, W. B. & Cobb, J. (1992) Cardiology 81, 291-298. - PubMed
-
- Hogan, P. G., Chen, L., Nardone, J. & Rao, A. (2003) Genes Dev. 17, 2205-2232. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

