Differential regulation of innate and adaptive immune responses in viral encephalitis
- PMID: 14972563
- PMCID: PMC7126141
- DOI: 10.1016/j.virol.2003.09.023
Differential regulation of innate and adaptive immune responses in viral encephalitis
Abstract
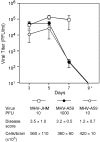
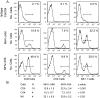
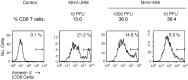
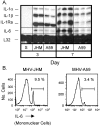
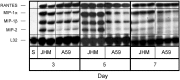
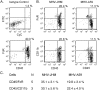
Viral encephalitis is a global health concern. The ability of a virus to modulate the immune response can have a pivotal effect on the course of disease and the fate of the infected host. In this study, we sought to understand the immunological basis for the fatal encephalitis following infection with the murine coronavirus, mouse hepatitis virus (MHV)-JHM, in contrast with the more attenuated MHV-A59. Distinct glial cell cytokine and chemokine response patterns were observed within 3 days after infection, became progressively more polarized during the course of infection and with the infiltration of leukocytes. In the brain, MHV-JHM infection induced strong accumulation of IFNbeta mRNA relative to IFNgamma mRNA. This trend was reversed in MHV-A59 infection and was accompanied by increased CD8 T cell infiltration into brain compared to MHV-JHM infection. Increased apoptosis appeared to contribute to the diminished presence of CD8 T cells in MHV-JHM-infected brain with the consequence of a lower potential for IFNgamma production and antiviral activity. MHV-JHM infection also induced sustained mRNA accumulation of the innate immune response products interleukin (IL)-6 and IL-1. Furthermore, high levels of macrophage-inflammatory protein (MIP)-1alpha, MIP-1beta, and MIP-2 mRNA were observed at the onset of MHV-JHM infection and correlated with a marked elevation in the number of macrophages in the brain on day 7 compared to MHV-A59 infection. These observations indicate that differences in the severity of viral encephalitis may reflect the differential ability of viruses to stimulate innate immune responses within the CNS and subsequently the character of infiltrating leukocyte populations.
Figures


Similar articles
-
Mouse hepatitis virus neurovirulence: evidence of a linkage between S glycoprotein expression and immunopathology.Virology. 2004 Jan 5;318(1):45-54. doi: 10.1016/j.virol.2003.08.041. Virology. 2004. PMID: 14972534 Free PMC article.
-
Both spike and background genes contribute to murine coronavirus neurovirulence.J Virol. 2006 Jul;80(14):6834-43. doi: 10.1128/JVI.00432-06. J Virol. 2006. PMID: 16809289 Free PMC article.
-
Preferential infection of mature dendritic cells by mouse hepatitis virus strain JHM.J Virol. 2006 Mar;80(5):2506-14. doi: 10.1128/JVI.80.5.2506-2514.2006. J Virol. 2006. PMID: 16474157 Free PMC article.
-
Pathogenesis of acute and chronic central nervous system infection with variants of mouse hepatitis virus, strain JHM.Immunol Res. 2007;39(1-3):160-72. doi: 10.1007/s12026-007-0079-y. Immunol Res. 2007. PMID: 17917063 Free PMC article. Review.
-
Murine coronavirus neuropathogenesis: determinants of virulence.J Neurovirol. 2010 Nov;16(6):427-34. doi: 10.3109/13550284.2010.529238. Epub 2010 Nov 12. J Neurovirol. 2010. PMID: 21073281 Free PMC article. Review.
Cited by
-
Replication of Rocio virus in primary cultures of mouse neural cells.Einstein (Sao Paulo). 2023 May 29;21:eAO0160. doi: 10.31744/einstein_journal/2023AO0160. eCollection 2023. Einstein (Sao Paulo). 2023. PMID: 37255058 Free PMC article.
-
Multi-phenotype analysis for enhanced classification of 11 herpes simplex virus 1 strains.J Gen Virol. 2022 Oct;103(10):001780. doi: 10.1099/jgv.0.001780. J Gen Virol. 2022. PMID: 36264606 Free PMC article.
-
Experimental Models of COVID-19.Front Cell Infect Microbiol. 2022 Jan 5;11:792584. doi: 10.3389/fcimb.2021.792584. eCollection 2021. Front Cell Infect Microbiol. 2022. PMID: 35096645 Free PMC article. Review.
-
Multi-Organ Histopathological Changes in a Mouse Hepatitis Virus Model of COVID-19.Viruses. 2021 Aug 27;13(9):1703. doi: 10.3390/v13091703. Viruses. 2021. PMID: 34578284 Free PMC article.
-
DJ-1-Nrf2 axis is activated upon murine β-coronavirus infection in the CNS.Brain Disord. 2021 Dec;4:100021. doi: 10.1016/j.dscb.2021.100021. Epub 2021 Sep 5. Brain Disord. 2021. PMID: 34514445 Free PMC article.
References
-
- Asensio V.C., Kincaid C., Campbell I.L. Chemokines and the inflammatory response to viral infection in the central nervous system with a focus on lymphocytic choriomeningitis virus. J. NeuroVirol. 1999;5:65–75. - PubMed
-
- Baggiolini M., Dewald B., Moser B. Interleukin-8 and related chemotactic cytokines—CXC and CC chemokines. Adv. Immunol. 1994;55:97–179. - PubMed
-
- Bergmann C.C., Yao Q., Lin M., Stohlman S.A. The JHM strain of mouse hepatitis virus induces a spike protein-specific Db-restricted cytotoxic T cell response. J. Gen. Virol. 1996;77:315–425. - PubMed
-
- Bergmann C.C., Altman J.D., Hinton D., Stohlman S.A. Inverted immunodominance and impaired cytolytic function of CD8+ T cells during viral persistence in the central nervous system. J. Immunol. 1999;163:3379–3387. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

