The v3 loop is accessible on the surface of most human immunodeficiency virus type 1 primary isolates and serves as a neutralization epitope
- PMID: 14963135
- PMCID: PMC369230
- DOI: 10.1128/jvi.78.5.2394-2404.2004
The v3 loop is accessible on the surface of most human immunodeficiency virus type 1 primary isolates and serves as a neutralization epitope
Abstract
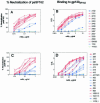
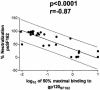
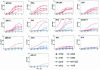
Antibodies (Abs) against the V3 loop of the human immunodeficiency virus type 1 gp120 envelope glycoprotein were initially considered to mediate only type-specific neutralization of T-cell-line-adapted viruses. However, recent data show that cross-neutralizing V3 Abs also exist, and primary isolates can be efficiently neutralized with anti-V3 monoclonal Abs (MAbs). The neutralizing activities of anti-V3 polyclonal Abs and MAbs may, however, be limited due to antigenic variations of the V3 region, a lack of V3 exposure on the surface of intact virions, or Ab specificity. For clarification of this issue, a panel of 32 human anti-V3 MAbs were screened for neutralization of an SF162-pseudotyped virus in a luciferase assay. MAbs selected with a V3 fusion protein whose V3 region mimics the conformation of the native virus were significantly more potent than MAbs selected with V3 peptides. Seven MAbs were further tested for neutralizing activity against 13 clade B viruses in a single-round peripheral blood mononuclear cell assay. While there was a spectrum of virus sensitivities to the anti-V3 MAbs observed, 12 of the 13 viruses were neutralized by one or more of the anti-V3 MAbs. MAb binding to intact virions correlated significantly with binding to solubilized gp120s and with the potency of neutralization. These results demonstrate that the V3 loop is accessible on the native virus envelope, that the strength of binding of anti-V3 Abs correlates with the potency of neutralization, that V3 epitopes may be shared rather than type specific, and that Abs against the V3 loop, particularly those targeting conformational epitopes, can mediate the neutralization of primary isolates.
Figures
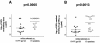

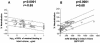
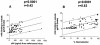
Similar articles
-
Human monoclonal antibodies specific for conformation-sensitive epitopes of V3 neutralize human immunodeficiency virus type 1 primary isolates from various clades.J Virol. 2002 Sep;76(18):9035-45. doi: 10.1128/jvi.76.18.9035-9045.2002. J Virol. 2002. PMID: 12186887 Free PMC article.
-
Conserved and exposed epitopes on intact, native, primary human immunodeficiency virus type 1 virions of group M.J Virol. 2000 Aug;74(15):7096-107. doi: 10.1128/jvi.74.15.7096-7107.2000. J Virol. 2000. PMID: 10888650 Free PMC article.
-
The cross-clade neutralizing activity of a human monoclonal antibody is determined by the GPGR V3 motif of HIV type 1.AIDS Res Hum Retroviruses. 2004 Nov;20(11):1254-8. doi: 10.1089/aid.2004.20.1254. AIDS Res Hum Retroviruses. 2004. PMID: 15588347
-
Neutralization of HIV-1: a paradox of humoral proportions.FASEB J. 1991 Jul;5(10):2437-55. doi: 10.1096/fasebj.5.10.1712328. FASEB J. 1991. PMID: 1712328 Review.
-
Passive immunization to prevent mother-infant transmission of human immunodeficiency virus: current issues and future directions.Pediatr Infect Dis J. 1991 Jun;10(6):456-62. doi: 10.1097/00006454-199106000-00009. Pediatr Infect Dis J. 1991. PMID: 1712936 Review.
Cited by
-
Functional Anatomy of the Trimer Apex Reveals Key Hydrophobic Constraints That Maintain the HIV-1 Envelope Spike in a Closed State.mBio. 2021 Mar 30;12(2):e00090-21. doi: 10.1128/mBio.00090-21. mBio. 2021. PMID: 33785631 Free PMC article.
-
Human anti-V3 HIV-1 monoclonal antibodies encoded by the VH5-51/VL lambda genes define a conserved antigenic structure.PLoS One. 2011;6(12):e27780. doi: 10.1371/journal.pone.0027780. Epub 2011 Dec 2. PLoS One. 2011. PMID: 22164215 Free PMC article.
-
Identification of a new quaternary neutralizing epitope on human immunodeficiency virus type 1 virus particles.J Virol. 2005 Apr;79(8):5232-7. doi: 10.1128/JVI.79.8.5232-5237.2005. J Virol. 2005. PMID: 15795308 Free PMC article.
-
Design of immunogens that present the crown of the HIV-1 V3 loop in a conformation competent to generate 447-52D-like antibodies.Biochem J. 2006 Nov 1;399(3):483-91. doi: 10.1042/BJ20060588. Biochem J. 2006. PMID: 16827663 Free PMC article.
-
Inducing cross-clade neutralizing antibodies against HIV-1 by immunofocusing.PLoS One. 2008;3(12):e3937. doi: 10.1371/journal.pone.0003937. Epub 2008 Dec 15. PLoS One. 2008. PMID: 19081789 Free PMC article.
References
-
- Ahlers, J. D., C. D. Pendleton, N. Dunlop, A. Minassian, P. L. Nara, and J. A. Berzofsky. 1993. Construction of an HIV-1 peptide vaccine containing a multideterminant helper peptide linked to a V3 loop peptide 18 inducing strong neutralizing antibody responses in mice of multiple MHC haplotypes after two immunizations. J. Immunol. 150:5647-5665. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

