Vaccinia virus entry into cells is dependent on a virion surface protein encoded by the A28L gene
- PMID: 14963132
- PMCID: PMC369249
- DOI: 10.1128/jvi.78.5.2357-2366.2004
Vaccinia virus entry into cells is dependent on a virion surface protein encoded by the A28L gene
Abstract
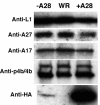
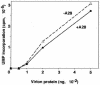
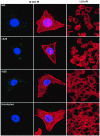
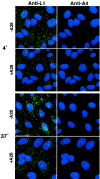
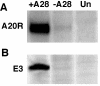
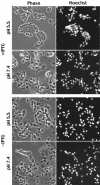
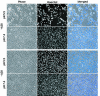
The A28L gene of vaccinia virus is conserved in all poxviruses and encodes a protein that is anchored to the surface of infectious intracellular mature virions (IMV) and consequently lies beneath the additional envelope of extracellular virions. A conditional lethal recombinant vaccinia virus, vA28-HAi, with an inducible A28L gene, undergoes a single round of replication in the absence of inducer, producing IMV, as well as extracellular virions with actin tails, but fails to infect neighboring cells. We show here that purified A28-deficient IMV appeared to be indistinguishable from wild-type IMV and were competent to synthesize RNA in vitro. Nevertheless, A28-deficient virions did not induce cytopathic effects, express early genes, or initiate a productive infection. Although A28-deficient IMV bound to the surface of cells, their cores did not penetrate into the cytoplasm. An associated defect in membrane fusion was demonstrated by the failure of low pH to trigger syncytium formation when cells were infected with vA28-HAi in the absence of inducer (fusion from within) or when cells were incubated with a high multiplicity of A28-deficient virions (fusion from without). The correlation between the entry block and the inability of A28-deficient virions to mediate fusion provided compelling evidence for a relationship between these events. Because repression of A28 inhibited cell-to-cell spread, which is mediated by extracellular virions, all forms of vaccinia virus regardless of their outer coat must use a common A28-dependent mechanism of cell penetration. Furthermore, since A28 is conserved, all poxviruses are likely to penetrate cells in a similar way.
Figures

Similar articles
-
Vaccinia virus A21 virion membrane protein is required for cell entry and fusion.J Virol. 2005 Aug;79(15):9458-69. doi: 10.1128/JVI.79.15.9458-9469.2005. J Virol. 2005. PMID: 16014909 Free PMC article.
-
Vaccinia virus A28L gene encodes an essential protein component of the virion membrane with intramolecular disulfide bonds formed by the viral cytoplasmic redox pathway.J Virol. 2004 Mar;78(5):2348-56. doi: 10.1128/jvi.78.5.2348-2356.2004. J Virol. 2004. PMID: 14963131 Free PMC article.
-
Vaccinia virus H2 protein is an essential component of a complex involved in virus entry and cell-cell fusion.J Virol. 2005 Apr;79(8):4744-54. doi: 10.1128/JVI.79.8.4744-4754.2005. J Virol. 2005. PMID: 15795260 Free PMC article.
-
The exit of vaccinia virus from infected cells.Virus Res. 2004 Dec;106(2):189-97. doi: 10.1016/j.virusres.2004.08.015. Virus Res. 2004. PMID: 15567497 Review.
-
The formation and function of extracellular enveloped vaccinia virus.J Gen Virol. 2002 Dec;83(Pt 12):2915-2931. doi: 10.1099/0022-1317-83-12-2915. J Gen Virol. 2002. PMID: 12466468 Review.
Cited by
-
Association of vaccinia virus fusion regulatory proteins with the multicomponent entry/fusion complex.J Virol. 2007 Jun;81(12):6286-93. doi: 10.1128/JVI.00274-07. Epub 2007 Apr 4. J Virol. 2007. PMID: 17409143 Free PMC article.
-
Vaccinia virus A21 virion membrane protein is required for cell entry and fusion.J Virol. 2005 Aug;79(15):9458-69. doi: 10.1128/JVI.79.15.9458-9469.2005. J Virol. 2005. PMID: 16014909 Free PMC article.
-
Vaccinia virus l1 protein is required for cell entry and membrane fusion.J Virol. 2008 Sep;82(17):8687-94. doi: 10.1128/JVI.00852-08. Epub 2008 Jul 2. J Virol. 2008. PMID: 18596103 Free PMC article.
-
Vaccinia virus A56/K2 fusion regulatory protein interacts with the A16 and G9 subunits of the entry fusion complex.J Virol. 2008 Jun;82(11):5153-60. doi: 10.1128/JVI.00162-08. Epub 2008 Mar 19. J Virol. 2008. PMID: 18353946 Free PMC article.
-
Inhibition of Vaccinia virus entry by a broad spectrum antiviral peptide.Virology. 2009 Jun 5;388(2):248-59. doi: 10.1016/j.virol.2009.03.023. Epub 2009 Apr 22. Virology. 2009. PMID: 19395056 Free PMC article.
References
-
- Bablanian, R., B. Baxt, J. A. Sonnabend, and M. Esteban. 1978. Studies on the mechanisms of vaccinia virus cytopathic effects. II. Early cell rounding is associated with virus polypeptide synthesis. J. Gen. Virol. 39:403-413. - PubMed
-
- Boulter, E. A., and G. Appleyard. 1973. Differences between extracellular and intracellular forms of poxvirus and their implications. Prog. Med. Virol. 16:86-108. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

