Non-proteolytic inactivation of geminin requires CDK-dependent ubiquitination
- PMID: 14767479
- PMCID: PMC2691133
- DOI: 10.1038/ncb1100
Non-proteolytic inactivation of geminin requires CDK-dependent ubiquitination
Abstract
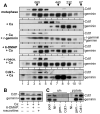
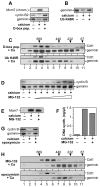
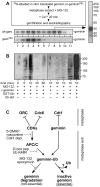
In late mitosis and G1, a complex of the essential initiation proteins Mcm2-7 are assembled onto replication origins to 'license' them for initiation. At other times licensing is inhibited by cyclin-dependent kinases (CDKs) and geminin, thus ensuring that origins fire only once per cell cycle. Here we show that, paradoxically, CDKs are also required to inactivate geminin and activate the licensing system. On exit from metaphase in Xenopus laevis egg extracts, CDK-dependent activation of the anaphase-promoting complex (APC/C) results in the transient polyubiquitination of geminin. This ubiquitination triggers geminin inactivation without requiring ubiquitin-dependent proteolysis, and is essential for replication origins to become licensed. This reveals an unexpected role for CDKs and ubiquitination in activating chromosomal DNA replication.
Figures


Similar articles
-
Negative regulation of geminin by CDK-dependent ubiquitination controls replication licensing.Cell Cycle. 2004 Apr;3(4):443-5. Epub 2004 Apr 1. Cell Cycle. 2004. PMID: 15004531 Free PMC article.
-
Evidence for a mammalian late-G1 phase inhibitor of replication licensing distinct from geminin or Cdk activity.Nucleus. 2011 Sep-Oct;2(5):455-64. doi: 10.4161/nucl.2.5.17859. Epub 2011 Sep 1. Nucleus. 2011. PMID: 21983086 Free PMC article.
-
Geminin, an inhibitor of DNA replication, is degraded during mitosis.Cell. 1998 Jun 12;93(6):1043-53. doi: 10.1016/s0092-8674(00)81209-x. Cell. 1998. PMID: 9635433
-
Control of DNA replication licensing in a cell cycle.Genes Cells. 2002 Jun;7(6):523-34. doi: 10.1046/j.1365-2443.2002.00544.x. Genes Cells. 2002. PMID: 12059957 Review.
-
Regulation of early events in chromosome replication.Curr Biol. 2004 Sep 21;14(18):R778-86. doi: 10.1016/j.cub.2004.09.019. Curr Biol. 2004. PMID: 15380092 Review.
Cited by
-
Behavior of replication origins in Eukaryota - spatio-temporal dynamics of licensing and firing.Cell Cycle. 2015;14(14):2251-64. doi: 10.1080/15384101.2015.1056421. Epub 2015 Jun 1. Cell Cycle. 2015. PMID: 26030591 Free PMC article. Review.
-
Regulation of replication licensing by acetyltransferase Hbo1.Mol Cell Biol. 2006 Feb;26(3):1098-108. doi: 10.1128/MCB.26.3.1098-1108.2006. Mol Cell Biol. 2006. PMID: 16428461 Free PMC article.
-
Dynamic interactions of high Cdt1 and geminin levels regulate S phase in early Xenopus embryos.Development. 2012 Jan;139(1):63-74. doi: 10.1242/dev.068676. Epub 2011 Nov 17. Development. 2012. PMID: 22096080 Free PMC article.
-
MAP kinase dependent cyclinE/cdk2 activity promotes DNA replication in early sea urchin embryos.Dev Biol. 2009 Oct 15;334(2):383-94. doi: 10.1016/j.ydbio.2009.07.043. Epub 2009 Aug 6. Dev Biol. 2009. PMID: 19665013 Free PMC article.
-
Cdt1 revisited: complex and tight regulation during the cell cycle and consequences of deregulation in mammalian cells.Cell Div. 2006 Oct 17;1:22. doi: 10.1186/1747-1028-1-22. Cell Div. 2006. PMID: 17042960 Free PMC article.
References
-
- Diffley JFX. Building the perfect switch. Current Biol. 2001;11:R367–370. - PubMed
-
- Nishitani H, Lygerou Z. Control of DNA replication licensing in a cell cycle. Genes Cells. 2002;7:523–534. - PubMed
-
- Chong JP, Mahbubani HM, Khoo CY, Blow JJ. Purification of an MCM-containing complex as a component of the DNA replication licensing system. Nature. 1995;375:418–421. - PubMed
-
- Kubota Y, Mimura S, Nishimoto S, Takisawa H, Nojima H. Identification of the yeast MCM3-related protein as a component of Xenopus DNA replication licensing factor. Cell. 1995;81:601–609. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

