Glycine decarboxylase mediates a postbinding step in duck hepatitis B virus infection
- PMID: 14747552
- PMCID: PMC369508
- DOI: 10.1128/jvi.78.4.1873-1881.2004
Glycine decarboxylase mediates a postbinding step in duck hepatitis B virus infection
Abstract
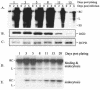
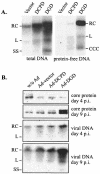
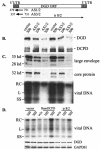
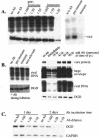
Envelope protein precursors of many viruses are processed by a basic endopeptidase to generate two molecules, one for receptor binding and the other for membrane fusion. Such a cleavage event has not been demonstrated for the hepatitis B virus family. Two binding partners for duck hepatitis B virus (DHBV) pre-S envelope protein have been identified. Duck carboxypeptidase D (DCPD) interacts with the full-length pre-S protein and is the DHBV docking receptor, while duck glycine decarboxylase (DGD) has the potential to bind several deletion constructs of the pre-S protein in vitro. Interestingly, DGD but not DCPD expression was diminished following prolonged culture of primary duck hepatocytes (PDH), which impaired productive DHBV infection. Introduction of exogenous DGD promoted formation of protein-free viral genome, suggesting restoration of several early events in viral life cycle. Conversely, blocking DGD expression in fresh PDH by antisense RNA abolished DHBV infection. Moreover, addition of DGD antibodies soon after virus binding reduced endogenous DGD protein levels and impaired production of covalently closed circular DNA, the template for DHBV gene expression and genome replication. Our findings implicate this second pre-S binding protein as a critical cellular factor for productive DHBV infection. We hypothesize that DCPD, a molecule cycling between the cell surface and the trans-Golgi network, targets DHBV particles to the secretary pathway for proteolytic cleavage of viral envelope protein. DGD represents the functional equivalent of other virus receptors in its interaction with processed viral particles.
Figures


Similar articles
-
Initiation of duck hepatitis B virus infection requires cleavage by a furin-like protease.J Virol. 2010 May;84(9):4569-78. doi: 10.1128/JVI.02281-09. Epub 2010 Feb 24. J Virol. 2010. PMID: 20181690 Free PMC article.
-
Identification and expression of glycine decarboxylase (p120) as a duck hepatitis B virus pre-S envelope-binding protein.J Biol Chem. 1999 Sep 24;274(39):27658-65. doi: 10.1074/jbc.274.39.27658. J Biol Chem. 1999. PMID: 10488106
-
Covalently closed circular DNA is the predominant form of duck hepatitis B virus DNA that persists following transient infection.J Virol. 2005 Oct;79(19):12242-52. doi: 10.1128/JVI.79.19.12242-12252.2005. J Virol. 2005. PMID: 16160150 Free PMC article.
-
Viral and cellular determinants involved in hepadnaviral entry.World J Gastroenterol. 2007 Jan 7;13(1):22-38. doi: 10.3748/wjg.v13.i1.22. World J Gastroenterol. 2007. PMID: 17206752 Free PMC article. Review.
-
From DCPD to NTCP: the long journey towards identifying a functional hepatitis B virus receptor.Clin Mol Hepatol. 2015 Sep;21(3):193-9. doi: 10.3350/cmh.2015.21.3.193. Epub 2015 Sep 30. Clin Mol Hepatol. 2015. PMID: 26523264 Free PMC article. Review.
Cited by
-
Heterologous replacement of the supposed host determining region of avihepadnaviruses: high in vivo infectivity despite low infectivity for hepatocytes.PLoS Pathog. 2008 Dec;4(12):e1000230. doi: 10.1371/journal.ppat.1000230. Epub 2008 Dec 5. PLoS Pathog. 2008. PMID: 19057662 Free PMC article.
-
Identification of NTCP as an HBV receptor: the beginning of the end or the end of the beginning?Gastroenterology. 2014 Apr;146(4):902-5. doi: 10.1053/j.gastro.2014.02.024. Epub 2014 Feb 24. Gastroenterology. 2014. PMID: 24576732 Free PMC article.
-
Characterization of genotype-specific carboxyl-terminal cleavage sites of hepatitis B virus e antigen precursor and identification of furin as the candidate enzyme.J Virol. 2009 Apr;83(8):3507-17. doi: 10.1128/JVI.02348-08. Epub 2009 Feb 4. J Virol. 2009. PMID: 19193799 Free PMC article.
-
Initiation of duck hepatitis B virus infection requires cleavage by a furin-like protease.J Virol. 2010 May;84(9):4569-78. doi: 10.1128/JVI.02281-09. Epub 2010 Feb 24. J Virol. 2010. PMID: 20181690 Free PMC article.
-
Hepatitis B virus-cell interactions and pathogenesis.J Cell Physiol. 2008 Aug;216(2):289-94. doi: 10.1002/jcp.21416. J Cell Physiol. 2008. PMID: 18302164 Free PMC article. Review.
References
-
- Chassot, S., V. Lambert, A. Kay, C. Godinot, B. Roux, C. Trepo, and L. Cova. 1993. Fine mapping of neutralization epitopes on duck hepatitis B virus (DHBV) pre-S protein using monoclonal antibodies and overlapping peptides. Virology 192:217-223. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

