Gene therapy approaches for bone regeneration
- PMID: 14745239
- PMCID: PMC3565156
- DOI: 10.1159/000075031
Gene therapy approaches for bone regeneration
Abstract
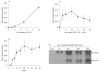
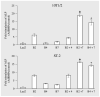
Gene therapy represents a promising approach for delivering regenerative molecules to specific tissues including bone. Several laboratories have shown that virus-based BMP expression vectors can stimulate osteoblast differentiation and bone formation in vivo. Both in vivo and ex vivo transduction of cells can induce bone formation at ectopic and orthotopic sites. Adenovirus and direct DNA delivery of genes encoding regenerative molecules can heal critical-sized defects of cranial and long bones. Although osteogenic activity can be demonstrated for individual BMP vectors, substantial synergies may be achieved using combinatorial gene therapy to express complimentary osteogenic signals including specific combinations of BMPs or BMPs and transcription factors. Further control of the bone regeneration process may also be achieved through the use of inducible promoters that can be used to control the timing and magnitude of expression for a particular gene. Using these types of approaches, it should be possible to mimic natural processes of bone development and fracture repair and, in so doing, be able to precisely control both the amount and type of bone regenerated.
Copyright 2004 S. Karger AG, Basel
Figures




Similar articles
-
Combinatorial gene therapy with BMP2/7 enhances cranial bone regeneration.J Dent Res. 2008 Sep;87(9):845-9. doi: 10.1177/154405910808700906. J Dent Res. 2008. PMID: 18719211 Free PMC article.
-
Characterization of the distinct orthotopic bone-forming activity of 14 BMPs using recombinant adenovirus-mediated gene delivery.Gene Ther. 2004 Sep;11(17):1312-20. doi: 10.1038/sj.gt.3302298. Gene Ther. 2004. PMID: 15269709
-
Ex vivo gene therapy for skeletal regeneration in cranial defects compromised by postoperative radiotherapy.Hum Gene Ther. 2003 Jul 20;14(11):1107-15. doi: 10.1089/104303403322124819. Hum Gene Ther. 2003. PMID: 12885349
-
Bone morphogenetic proteins.Growth Factors. 2004 Dec;22(4):233-41. doi: 10.1080/08977190412331279890. Growth Factors. 2004. PMID: 15621726 Review.
-
Non-viral gene therapy for bone tissue engineering.Biotechnol Genet Eng Rev. 2013;29:206-20. doi: 10.1080/02648725.2013.801227. Epub 2013 Aug 2. Biotechnol Genet Eng Rev. 2013. PMID: 24568281 Review.
Cited by
-
Current concepts of bone grafting in trauma surgery.J Clin Orthop Trauma. 2023 Aug 5;43:102231. doi: 10.1016/j.jcot.2023.102231. eCollection 2023 Aug. J Clin Orthop Trauma. 2023. PMID: 37636005 Free PMC article.
-
Percutaneous nonviral delivery of hepatocyte growth factor in an osteotomy gap promotes bone repair in rabbits: a preliminary study.Clin Orthop Relat Res. 2008 Dec;466(12):2962-72. doi: 10.1007/s11999-008-0493-z. Epub 2008 Sep 24. Clin Orthop Relat Res. 2008. PMID: 18813894 Free PMC article.
-
Combinatorial gene therapy with BMP2/7 enhances cranial bone regeneration.J Dent Res. 2008 Sep;87(9):845-9. doi: 10.1177/154405910808700906. J Dent Res. 2008. PMID: 18719211 Free PMC article.
-
Foxo1, a novel regulator of osteoblast differentiation and skeletogenesis.J Biol Chem. 2010 Oct 1;285(40):31055-65. doi: 10.1074/jbc.M109.079962. Epub 2010 Jul 22. J Biol Chem. 2010. PMID: 20650891 Free PMC article.
-
PDGF-BB and IL-4 co-overexpression is a potential strategy to enhance mesenchymal stem cell-based bone regeneration.Stem Cell Res Ther. 2021 Jan 7;12(1):40. doi: 10.1186/s13287-020-02086-8. Stem Cell Res Ther. 2021. PMID: 33413614 Free PMC article.
References
-
- Baltzer AW, Lattermann C, Whalen JD, Wooley P, Weiss K, Grimm M, Ghivizzani SC, Robbins PD, Evans CH. Genetic enhancement of fracture repair: Healing of an experimental segmental defect by adenoviral transfer of the BMP-2 gene. Gene Ther. 2000;7:734–739. - PubMed
-
- Bergelson JM, Cunningham JA, Droguett G, Kurt-Jones EA, Krithivas A, Hong JS, Horwitz MS, Crowell RL, Finberg RW. Isolation of a common receptor for Coxsackie B viruses and adenoviruses 2 and 5. Science. 1997;275:1320–1323. - PubMed
-
- Bonadio J, Smiley E, Patil P, Goldstein S. Localized, direct plasmid gene delivery in vivo: Prolonged therapy results in reproducible tissue regeneration. Nat Med. 1999;5:753–759. - PubMed
-
- Ducy P, Schinke T, Karsenty G. The osteoblast: A sophisticated fibroblast under central surveillance. Science. 2000;289:1501–1504. - PubMed
-
- Ducy P, Zhang R, Geoffroy V, Ridall AL, Karsenty G. Osf2/Cbfa1: A transcriptional activator of osteoblast differentiation. Cell. 1997;89:747–754. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

