Identification of the protein storage vacuole and protein targeting to the vacuole in leaf cells of three plant species
- PMID: 14730078
- PMCID: PMC344539
- DOI: 10.1104/pp.103.030635
Identification of the protein storage vacuole and protein targeting to the vacuole in leaf cells of three plant species
Abstract
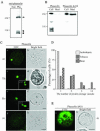
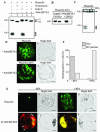
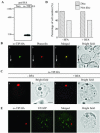
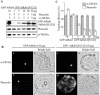
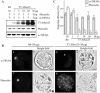
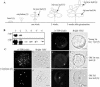
Protein storage vacuoles (PSVs) are specialized vacuoles devoted to the accumulation of large amounts of protein in the storage tissues of plants. In this study, we investigated the presence of the storage vacuole and protein trafficking to the compartment in cells of tobacco (Nicotiana tabacum), common bean (Phaseolus vulgaris), and Arabidopsis leaf tissue. When we expressed phaseolin, the major storage protein of common bean, or an epitope-tagged version of alpha-tonoplast intrinsic protein (alpha-TIP, a tonoplast aquaporin of PSV), in protoplasts derived from leaf tissues, these proteins were targeted to a compartment ranging in size from 2 to 5 microm in all three plant species. Most Arabidopsis leaf cells have one of these organelles. In contrast, from one to five these organelles occurred in bean and tobacco leaf cells. Also, endogenous alpha-TIP is localized in a similar compartment in untransformed leaf cells of common bean and is colocalized with transiently expressed epitope-tagged alpha-TIP. In Arabidopsis, phaseolin contained N-glycans modified by Golgi enzymes and its traffic was sensitive to brefeldin A. However, trafficking of alpha-TIP was insensitive to brefeldin A treatment and was not affected by the dominant-negative mutant of AtRab1. In addition, a modified alpha-TIP with an insertion of an N-glycosylation site has the endoplasmic reticulum-type glycans. Finally, the early step of phaseolin traffic, from the endoplasmic reticulum to the Golgi complex, required the activity of the small GTPase Sar1p, a key component of coat protein complex II-coated vesicles, independent of the presence of the vacuolar sorting signal in phaseolin. Based on these results, we propose that the proteins we analyzed are targeted to the PSV or equivalent organelle in leaf cells and that proteins can be transported to the PSV by two different pathways, the Golgi-dependent and Golgi-independent pathways, depending on the individual cargo proteins.
Figures



Similar articles
-
AtRMR1 functions as a cargo receptor for protein trafficking to the protein storage vacuole.J Cell Biol. 2005 Aug 29;170(5):757-67. doi: 10.1083/jcb.200504112. Epub 2005 Aug 22. J Cell Biol. 2005. PMID: 16115960 Free PMC article.
-
AtRabF2b (Ara7) acts on the vacuolar trafficking pathway in tobacco leaf epidermal cells.J Cell Sci. 2004 Dec 15;117(Pt 26):6377-89. doi: 10.1242/jcs.01564. Epub 2004 Nov 23. J Cell Sci. 2004. PMID: 15561767
-
Integral membrane protein sorting to vacuoles in plant cells: evidence for two pathways.J Cell Biol. 1998 Nov 30;143(5):1183-99. doi: 10.1083/jcb.143.5.1183. J Cell Biol. 1998. PMID: 9832548 Free PMC article.
-
Targeting of tonoplast proteins to the vacuole.Plant Sci. 2013 Oct;211:132-6. doi: 10.1016/j.plantsci.2013.07.005. Epub 2013 Jul 18. Plant Sci. 2013. PMID: 23987818 Review.
-
Endocytic and autophagic pathways crosstalk in plants.Curr Opin Plant Biol. 2015 Dec;28:39-47. doi: 10.1016/j.pbi.2015.08.010. Epub 2015 Oct 24. Curr Opin Plant Biol. 2015. PMID: 26453966 Review.
Cited by
-
Arabidopsis EPSIN1 plays an important role in vacuolar trafficking of soluble cargo proteins in plant cells via interactions with clathrin, AP-1, VTI11, and VSR1.Plant Cell. 2006 Sep;18(9):2258-74. doi: 10.1105/tpc.105.039123. Epub 2006 Aug 11. Plant Cell. 2006. PMID: 16905657 Free PMC article.
-
Preventing unintended proteolysis in plant protein biofactories.Plant Biotechnol J. 2008 Sep;6(7):633-48. doi: 10.1111/j.1467-7652.2008.00344.x. Epub 2008 Apr 28. Plant Biotechnol J. 2008. PMID: 18452504 Free PMC article. Review.
-
What is moving in the secretory pathway of plants?Plant Physiol. 2008 Aug;147(4):1493-503. doi: 10.1104/pp.108.124552. Plant Physiol. 2008. PMID: 18678741 Free PMC article. Review. No abstract available.
-
Contribution of chitinase A's C-terminal vacuolar sorting determinant to the study of soluble protein compartmentation.Int J Mol Sci. 2014 Jun 18;15(6):11030-9. doi: 10.3390/ijms150611030. Int J Mol Sci. 2014. PMID: 24945312 Free PMC article. Review.
-
Dengue virus E glycoprotein production in transgenic rice callus.Mol Biotechnol. 2014 Dec;56(12):1069-78. doi: 10.1007/s12033-014-9787-4. Mol Biotechnol. 2014. PMID: 25069989
References
-
- Aubert S, Gout E, Bligny R, Marty-Mazars D, Barrieu F, Alabouvette J, Marty F, Douce R (1996) Ultrastructural and biochemical characterization of autophagy in higher plant cells subjected to carbon deprivation: control by the supply of mitochondria with respiratory substrates. J Cell Biol 133: 1251–1263 - PMC - PubMed
-
- Bassham DC, Raikhel NV (2000) Unique features of the plant vacuolar sorting machinery. Curr Opin Cell Biol 12: 491–495 - PubMed
-
- Boevink P, Martin B, Oparka K, Santa Cruz S, Hawes C (1999) Transport of virally expressed green fluorescent protein through the secretory pathway in tobacco leaves is inhibited by cold shock and brefeldin A. Planta 208: 392–400
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

