GADD34-PP1c recruited by Smad7 dephosphorylates TGFbeta type I receptor
- PMID: 14718519
- PMCID: PMC2172339
- DOI: 10.1083/jcb.200307151
GADD34-PP1c recruited by Smad7 dephosphorylates TGFbeta type I receptor
Abstract
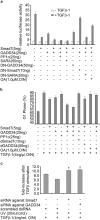
The cascade of phosphorylation is a pivotal event in transforming growth factor beta (TGFbeta) signaling. Reversible phosphorylation regulates fundamental aspects of cell activity. TGFbeta-induced Smad7 binds to type I receptor (TGFbeta type I receptor; TbetaRI) functioning as a receptor kinase antagonist. We found Smad7 interacts with growth arrest and DNA damage protein, GADD34, a regulatory subunit of the protein phosphatase 1 (PP1) holoenzyme, which subsequently recruits catalytic subunit of PP1 (PP1c) to dephosphorylate TbetaRI. Blocking Smad7 expression by RNA interference inhibits association of GADD34-PP1c complex with TbetaRI, indicating Smad7 acts as an adaptor protein in the formation of the PP1 holoenzyme that targets TbetaRI for dephosphorylation. SARA (Smad anchor for receptor activation) enhances the recruitment PP1c to the Smad7-GADD34 complex by controlling the specific subcellular localization of PP1c. Importantly, GADD34-PP1c recruited by Smad7 inhibits TGFbeta-induced cell cycle arrest and mediates TGFbeta resistance in responding to UV light irradiation. The dephosphorylation of TbetaRI mediated by Smad7 is an effective mechanism for governing negative feedback in TGFbeta signaling.
Figures






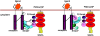
Similar articles
-
PP1 binds Sara and negatively regulates Dpp signaling in Drosophila melanogaster.Nat Genet. 2002 Aug;31(4):419-23. doi: 10.1038/ng938. Epub 2002 Jul 22. Nat Genet. 2002. PMID: 12134149
-
Yes-associated protein (YAP65) interacts with Smad7 and potentiates its inhibitory activity against TGF-beta/Smad signaling.Oncogene. 2002 Jul 25;21(32):4879-84. doi: 10.1038/sj.onc.1205623. Oncogene. 2002. PMID: 12118366
-
Type I transforming growth factor beta receptor binds to and activates phosphatidylinositol 3-kinase.J Biol Chem. 2005 Mar 18;280(11):10870-6. doi: 10.1074/jbc.M413223200. Epub 2005 Jan 18. J Biol Chem. 2005. PMID: 15657037
-
Functional analysis of the TGFbeta receptor/Smad pathway through gene ablation in mice.Int J Dev Biol. 2000 Apr;44(3):253-65. Int J Dev Biol. 2000. PMID: 10853822 Review.
-
Protein phosphatase 1--targeted in many directions.J Cell Sci. 2002 Jan 15;115(Pt 2):241-56. doi: 10.1242/jcs.115.2.241. J Cell Sci. 2002. PMID: 11839776 Review.
Cited by
-
The integrated stress response.EMBO Rep. 2016 Oct;17(10):1374-1395. doi: 10.15252/embr.201642195. Epub 2016 Sep 14. EMBO Rep. 2016. PMID: 27629041 Free PMC article. Review.
-
Tollip, an intracellular trafficking protein, is a novel modulator of the transforming growth factor-β signaling pathway.J Biol Chem. 2012 Nov 16;287(47):39653-63. doi: 10.1074/jbc.M112.388009. Epub 2012 Oct 1. J Biol Chem. 2012. PMID: 23027871 Free PMC article.
-
Protein phosphatase 1α interacting proteins in the human brain.OMICS. 2012 Jan-Feb;16(1-2):3-17. doi: 10.1089/omi.2011.0041. OMICS. 2012. PMID: 22321011 Free PMC article.
-
Formin-dependent TGF-β signaling for epithelial to mesenchymal transition.Mol Biol Cell. 2018 Jun 15;29(12):1465-1475. doi: 10.1091/mbc.E17-05-0325. Epub 2018 Apr 18. Mol Biol Cell. 2018. PMID: 29668357 Free PMC article.
-
The PPP1R15 Family of eIF2-alpha Phosphatase Targeting Subunits (GADD34 and CReP).Int J Mol Sci. 2023 Dec 10;24(24):17321. doi: 10.3390/ijms242417321. Int J Mol Sci. 2023. PMID: 38139150 Free PMC article. Review.
References
-
- Abdollah, S., M. Macias-Silva, T. Tsukazaki, H. Hayashi, L. Attisano, and J.L. Wrana. 1997. TβRI phosphorylation of Smad2 on Ser465 and Ser467 is required for Smad2-Smad4 complex formation and signaling. J. Biol. Chem. 272:27678–27685. - PubMed
-
- Aggen, J.B., A.C. Nairn, and R. Chamberlin. 2000. Regulation of protein phosphatase-1. Chem. Biol. 7:R13–R23. - PubMed
-
- Attisano, L., and J.L. Wrana. 2002. Signal transduction by the TGF-β superfamily. Science. 296:1646–1647. - PubMed
-
- Bennett, D., and L. Alphey. 2002. PP1 binds Sara and negatively regulates Dpp signaling in Drosophila melanogaster. Nat. Genet. 31:419–423. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

