Production of resurgent current in NaV1.6-null Purkinje neurons by slowing sodium channel inactivation with beta-pompilidotoxin
- PMID: 14715935
- PMCID: PMC6729564
- DOI: 10.1523/JNEUROSCI.3807-03.2004
Production of resurgent current in NaV1.6-null Purkinje neurons by slowing sodium channel inactivation with beta-pompilidotoxin
Abstract
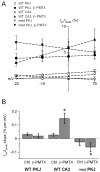
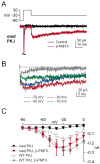
Voltage-gated tetrodotoxin-sensitive sodium channels of Purkinje neurons produce "resurgent" current with repolarization, which results from relief of an open-channel block that terminates current flow at positive potentials. The associated recovery of sodium channels from inactivation is thought to facilitate the rapid firing patterns characteristic of Purkinje neurons. Resurgent current appears to depend primarily on NaV1.6 alpha subunits, because it is greatly reduced in "med" mutant mice that lack NaV1.6. To identify factors that regulate the susceptibility of alpha subunits to open-channel block, we voltage clamped wild-type and med Purkinje neurons before and after slowing conventional inactivation with beta-pompilidotoxin (beta-PMTX). beta-PMTX increased resurgent current in wild-type neurons and induced resurgent current in med neurons. In med cells, the resurgent component of beta-PMTX-modified sodium currents could be selectively abolished by application of intracellular alkaline phosphatase, suggesting that, like in NaV1.6-expressing cells, the open-channel block of NaV1.1 and NaV1.2 subunits is regulated by constitutive phosphorylation. These results indicate that the endogenous blocker exists independently of NaV1.6 expression, and conventional inactivation regulates resurgent current by controlling the extent of open-channel block. In Purkinje cells, therefore, the relatively slow conventional inactivation kinetics of NaV1.6 appear well adapted to carry resurgent current. Nevertheless, NaV1.6 is not unique in its susceptibility to open-channel block, because under appropriate conditions, the non-NaV1.6 subunits can produce robust resurgent currents.
Figures



Similar articles
-
The contribution of resurgent sodium current to high-frequency firing in Purkinje neurons: an experimental and modeling study.J Neurosci. 2003 Jun 15;23(12):4899-912. doi: 10.1523/JNEUROSCI.23-12-04899.2003. J Neurosci. 2003. PMID: 12832512 Free PMC article.
-
A role for phosphorylation in the maintenance of resurgent sodium current in cerebellar purkinje neurons.J Neurosci. 2002 Apr 15;22(8):3100-7. doi: 10.1523/JNEUROSCI.22-08-03100.2002. J Neurosci. 2002. PMID: 11943813 Free PMC article.
-
The absence of resurgent sodium current in mouse spinal neurons.Brain Res. 1999 Dec 4;849(1-2):162-8. doi: 10.1016/s0006-8993(99)02060-0. Brain Res. 1999. PMID: 10592298
-
Cerebellum-related characteristics of Scn8a-mutant mice.Cerebellum. 2009 Sep;8(3):192-201. doi: 10.1007/s12311-009-0110-z. Epub 2009 May 8. Cerebellum. 2009. PMID: 19424768 Review.
-
Resurgent current of voltage-gated Na(+) channels.J Physiol. 2014 Nov 15;592(22):4825-38. doi: 10.1113/jphysiol.2014.277582. Epub 2014 Aug 28. J Physiol. 2014. PMID: 25172941 Free PMC article. Review.
Cited by
-
Human voltage-gated sodium channel mutations that cause inherited neuronal and muscle channelopathies increase resurgent sodium currents.J Clin Invest. 2010 Jan;120(1):369-78. doi: 10.1172/JCI40801. Epub 2009 Dec 28. J Clin Invest. 2010. PMID: 20038812 Free PMC article.
-
Na+ channel-dependent recruitment of Navβ4 to axon initial segments and nodes of Ranvier.J Neurosci. 2013 Apr 3;33(14):6191-202. doi: 10.1523/JNEUROSCI.4051-12.2013. J Neurosci. 2013. PMID: 23554500 Free PMC article.
-
Multisite phosphorylation of voltage-gated sodium channel alpha subunits from rat brain.J Proteome Res. 2010 Apr 5;9(4):1976-84. doi: 10.1021/pr901171q. J Proteome Res. 2010. PMID: 20131913 Free PMC article.
-
The Snake with the Scorpion's Sting: Novel Three-Finger Toxin Sodium Channel Activators from the Venom of the Long-Glanded Blue Coral Snake (Calliophis bivirgatus).Toxins (Basel). 2016 Oct 18;8(10):303. doi: 10.3390/toxins8100303. Toxins (Basel). 2016. PMID: 27763551 Free PMC article.
-
Modulation of neuronal sodium channels by the sea anemone peptide BDS-I.J Neurophysiol. 2012 Jun;107(11):3155-67. doi: 10.1152/jn.00785.2011. Epub 2012 Mar 21. J Neurophysiol. 2012. PMID: 22442564 Free PMC article.
References
-
- Burgess DL, Kohrman DC, Galt J, Plummer NW, Jones JM, Spear B, Meisler MH (1995) Mutation of a new sodium channel gene, Scn8a, in the mouse mutant “motor endplate disease.” Nat Genet 10: 461-465. - PubMed
-
- Do MT, Bean BP (2003) Subthreshold sodium currents and pacemaking of subthalamic neurons: modulation by slow inactivation. Neuron 39: 109-120. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
