Annexin II regulates fibrin homeostasis and neoangiogenesis in vivo
- PMID: 14702107
- PMCID: PMC300771
- DOI: 10.1172/JCI19684
Annexin II regulates fibrin homeostasis and neoangiogenesis in vivo
Abstract
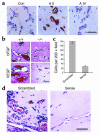
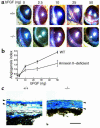
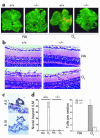
A central tenet of fibrinolysis is that tissue plasminogen activator-dependent (t-PA- dependent) conversion of plasminogen to active plasmin requires the presence of the cofactor/substrate fibrin. However, previous in vitro studies have suggested that the endothelial cell surface protein annexin II can stimulate t-PA-mediated plasminogen activation in the complete absence of fibrin. Here, homozygous annexin II-null mice displayed deposition of fibrin in the microvasculature and incomplete clearance of injury-induced arterial thrombi. While these animals demonstrated normal lysis of a fibrin-containing plasma clot, t-PA-dependent plasmin generation at the endothelial cell surface was markedly deficient. Directed migration of annexin II-null endothelial cells through fibrin and collagen lattices in vitro was also reduced, and an annexin II peptide mimicking sequences necessary for t-PA binding blocked endothelial cell invasion of Matrigel implants in wild-type mice. In addition, annexin II-deficient mice displayed markedly diminished neovascularization of fibroblast growth factor-stimulated cornea and of oxygen-primed neonatal retina. Capillary sprouting from annexin II-deficient aortic ring explants was markedly reduced in association with severe impairment of activation of metalloproteinase-9 and -13. These data establish annexin II as a regulator of cell surface plasmin generation and reveal that impaired endothelial cell fibrinolytic activity constitutes a barrier to effective neoangiogenesis.
Figures
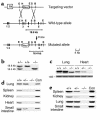
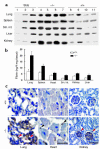
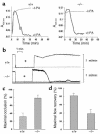
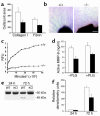
Similar articles
-
Regulation of fibrinolysis by S100A10 in vivo.Blood. 2011 Sep 15;118(11):3172-81. doi: 10.1182/blood-2011-05-353482. Epub 2011 Jul 18. Blood. 2011. PMID: 21768297
-
Hypoxia-inducible factor-1 drives annexin A2 system-mediated perivascular fibrin clearance in oxygen-induced retinopathy in mice.Blood. 2011 Sep 8;118(10):2918-29. doi: 10.1182/blood-2011-03-341214. Epub 2011 Jul 25. Blood. 2011. PMID: 21788340 Free PMC article.
-
Breast cancer cell surface annexin II induces cell migration and neoangiogenesis via tPA dependent plasmin generation.Exp Mol Pathol. 2010 Apr;88(2):278-86. doi: 10.1016/j.yexmp.2010.01.001. Epub 2010 Jan 15. Exp Mol Pathol. 2010. PMID: 20079732
-
Annexin II and regulation of cell surface fibrinolysis.Ann N Y Acad Sci. 2000 May;902:265-71. doi: 10.1111/j.1749-6632.2000.tb06321.x. Ann N Y Acad Sci. 2000. PMID: 10865846 Review.
-
Modulation of annexin II by homocysteine: implications for atherothrombosis.J Investig Med. 1998 Oct;46(8):364-9. J Investig Med. 1998. PMID: 9805420 Review.
Cited by
-
Genetic Modifiers of Stroke in Patients with Sickle Cell Disease-A Scoping Review.Int J Mol Sci. 2024 Jun 7;25(12):6317. doi: 10.3390/ijms25126317. Int J Mol Sci. 2024. PMID: 38928024 Free PMC article. Review.
-
The association of annexin A2 and cancers.Clin Transl Oncol. 2012 Sep;14(9):634-40. doi: 10.1007/s12094-012-0855-6. Epub 2012 Jul 24. Clin Transl Oncol. 2012. PMID: 22855149
-
Semaphorin 3D autocrine signaling mediates the metastatic role of annexin A2 in pancreatic cancer.Sci Signal. 2015 Aug 4;8(388):ra77. doi: 10.1126/scisignal.aaa5823. Sci Signal. 2015. PMID: 26243191 Free PMC article.
-
Dichotomous Role of Plasmin in Regulation of Macrophage Function after Acetaminophen Overdose.Am J Pathol. 2019 Oct;189(10):1986-2001. doi: 10.1016/j.ajpath.2019.07.003. Epub 2019 Aug 2. Am J Pathol. 2019. PMID: 31381887 Free PMC article.
-
Annexin-V stabilizes membrane defects by inducing lipid phase transition.Nat Commun. 2020 Jan 13;11(1):230. doi: 10.1038/s41467-019-14045-w. Nat Commun. 2020. PMID: 31932647 Free PMC article.
References
-
- Cines DB, et al. Endothelial cells in physiology and in the pathophysiology of vascular disorders. Blood. 1998;91:3527–3561. - PubMed
-
- Hajjar, K.A., Esmon, N.L., Marcus, A.J., and Muller, W.A. 2001. Vascular function in hemostasis. In Williams hematology. E. Beutler, M.A. Lichtman, B.S. Coller, T.J. Kipps, and U. Seligsohn, editors. McGraw-Hill. New York, New York, USA. 1451–1469.
-
- Enjyoji K, et al. Targeted disruption of CD39/ATP diphosphohydrolase results in disordered hemostasis and thromboregulation. Nat. Med. 1999;5:1010–1017. - PubMed
-
- Semeraro N, Colucci M. Tissue factor in health and disease. Thromb. Haemost. 1997;78:759–764. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

