A single injection of recombinant measles virus vaccines expressing human immunodeficiency virus (HIV) type 1 clade B envelope glycoproteins induces neutralizing antibodies and cellular immune responses to HIV
- PMID: 14671096
- PMCID: PMC303376
- DOI: 10.1128/jvi.78.1.146-157.2004
A single injection of recombinant measles virus vaccines expressing human immunodeficiency virus (HIV) type 1 clade B envelope glycoproteins induces neutralizing antibodies and cellular immune responses to HIV
Abstract
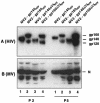
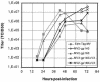
The anchored and secreted forms of the human immunodeficiency virus type 1 (HIV-1) 89.6 envelope glycoprotein, either complete or after deletion of the V3 loop, were expressed in a cloned attenuated measles virus (MV) vector. The recombinant viruses grew as efficiently as the parental virus and expressed high levels of the HIV protein. Expression was stable during serial passages. The immunogenicity of these recombinant vectors was tested in mice susceptible to MV and in macaques. High titers of antibodies to both MV and HIV-Env were obtained after a single injection in susceptible mice. These antibodies neutralized homologous SHIV89.6p virus, as well as several heterologous HIV-1 primary isolates. A gp160 mutant in which the V3 loop was deleted induced antibodies that neutralized heterologous viruses more efficiently than antibodies induced by the native envelope protein. A high level of CD8+ and CD4+ cells specific for HIV gp120 was also detected in MV-susceptible mice. Furthermore, recombinant MV was able to raise immune responses against HIV in mice and macaques with a preexisting anti-MV immunity. Therefore, recombinant MV vaccines inducing anti-HIV neutralizing antibodies and specific T lymphocytes responses deserve to be tested as a candidate AIDS vaccine.
Figures
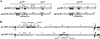
Similar articles
-
Antibody responses elicited in macaques immunized with human immunodeficiency virus type 1 (HIV-1) SF162-derived gp140 envelope immunogens: comparison with those elicited during homologous simian/human immunodeficiency virus SHIVSF162P4 and heterologous HIV-1 infection.J Virol. 2006 Sep;80(17):8745-62. doi: 10.1128/JVI.00956-06. J Virol. 2006. PMID: 16912322 Free PMC article.
-
Vaccination of rhesus macaques with recombinant Mycobacterium bovis bacillus Calmette-Guérin Env V3 elicits neutralizing antibody-mediated protection against simian-human immunodeficiency virus with a homologous but not a heterologous V3 motif.J Virol. 2005 Feb;79(3):1452-62. doi: 10.1128/JVI.79.3.1452-1462.2005. J Virol. 2005. PMID: 15650171 Free PMC article.
-
Evaluation of envelope vaccines derived from the South African subtype C human immunodeficiency virus type 1 TV1 strain.J Virol. 2005 Nov;79(21):13338-49. doi: 10.1128/JVI.79.21.13338-13349.2005. J Virol. 2005. PMID: 16227256 Free PMC article.
-
Elicitation of neutralizing antibodies with DNA vaccines expressing soluble stabilized human immunodeficiency virus type 1 envelope glycoprotein trimers conjugated to C3d.J Virol. 2004 May;78(9):4710-9. doi: 10.1128/jvi.78.9.4710-4719.2004. J Virol. 2004. PMID: 15078953 Free PMC article.
-
Screening of HIV-1 Env glycoproteins for the ability to raise neutralizing antibody using DNA immunization and recombinant vaccinia virus boosting.Virology. 1997 Apr 14;230(2):265-74. doi: 10.1006/viro.1997.8478. Virology. 1997. PMID: 9143282
Cited by
-
A safe and highly efficacious measles virus-based vaccine expressing SARS-CoV-2 stabilized prefusion spike.Proc Natl Acad Sci U S A. 2021 Mar 23;118(12):e2026153118. doi: 10.1073/pnas.2026153118. Proc Natl Acad Sci U S A. 2021. PMID: 33688034 Free PMC article.
-
Immunogenicity of next-generation HPV vaccines in non-human primates: Measles-vectored HPV vaccine versus Pichia pastoris recombinant protein vaccine.Vaccine. 2016 Sep 7;34(39):4724-4731. doi: 10.1016/j.vaccine.2016.07.051. Epub 2016 Aug 11. Vaccine. 2016. PMID: 27523740 Free PMC article.
-
An immune competent mouse model for the characterization of recombinant measles vaccines.Hum Vaccin Immunother. 2015;11(1):83-90. doi: 10.4161/hv.34358. Epub 2014 Nov 1. Hum Vaccin Immunother. 2015. PMID: 25483519 Free PMC article.
-
Experimental vaccines against potentially pandemic and highly pathogenic avian influenza viruses.Future Virol. 2013 Jan 1;8(1):25-41. doi: 10.2217/fvl.12.122. Future Virol. 2013. PMID: 23440999 Free PMC article.
-
Recombinant vectors as influenza vaccines.Curr Top Microbiol Immunol. 2009;333:243-67. doi: 10.1007/978-3-540-92165-3_13. Curr Top Microbiol Immunol. 2009. PMID: 19768410 Free PMC article. Review.
References
-
- Amara, R. R., F. Villinger, J. D. Altman, S. L. Lydy, S. P. O'Neil, S. I. Staprans, D. C. Montefiori, Y. Xu, J. G. Herndon, L. S. Wyatt, M. A. Candido, N. L. Kozyr, P. L. Earl, J. M. Smith, H. L. Ma, B. D. Grimm, M. L. Hulsey, J. Miller, H. M. McClure, J. M. McNicholl, B. Moss, and H. L. Robinson. 2001. Control of a mucosal challenge and prevention of AIDS by a multiprotein DNA/MVA vaccine. Science 292:69-74. - PubMed
-
- Andino, R., D. Silvera, S. D. Suggett, P. L. Achacoso, C. J. Miller, D. Baltimore, and M. B. Feinberg. 1994. Engineering poliovirus as a vaccine vector for the expression of diverse antigens. Science 265:1448-1451. - PubMed
-
- Baba, T. W., V. Liska, R. Hofmann-Lehmann, J. Vlasak, W. Xu, S. Ayehunie, L. A. Cavacini, M. R. Posner, H. Katinger, G. Stiegler, B. J. Bernacky, T. A. Rizvi, R. Schmidt, L. R. Hill, M. E. Keeling, Y. Lu, J. E. Wright, T. C. Chou, and R. M. Ruprecht. 2000. Human neutralizing monoclonal antibodies of the IgG1 subtype protect against mucosal simian-human immunodeficiency virus infection. Nat. Med. 6:200-206. - PubMed
-
- Baba, T. W., V. Liska, A. Khimani, N. Ray, P. Dailey, D. Penninck, R. Bronson, M. Greene, M. HM, M. LN, and R. Rprecht. 1999. Live attenuated, mulitiply deleted simian immunodeficiency viruses causes AIDS in infant and adult macaques. Nat. Med. 5:194-203. - PubMed
-
- Barnett, S. W., S. Lu, I. Srivastava, S. Cherpelis, A. Gettie, J. Blanchard, S. Wang, I. Mboudjeka, L. Leung, Y. Lian, A. Fong, C. Buckner, A. Ly, S. Hilt, J. Ulmer, C. T. Wild, J. R. Mascola, and L. Stamatatos. 2001. The ability of an oligomeric human immunodeficiency virus type 1 (HIV-1) envelope antigen to elicit neutralizing antibodies against primary HIV-1 isolates is improved following partial deletion of the second hypervariable region. J. Virol. 75:5526-5540. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

