Motility of dendritic spines in visual cortex in vivo: changes during the critical period and effects of visual deprivation
- PMID: 14663137
- PMCID: PMC307686
- DOI: 10.1073/pnas.2636949100
Motility of dendritic spines in visual cortex in vivo: changes during the critical period and effects of visual deprivation
Erratum in
- Proc Natl Acad Sci U S A. 2004 Mar 16;101(11):3991
Abstract
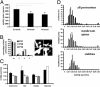
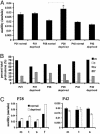
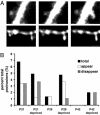
Cortical dendritic spines are highly motile postsynaptic structures onto which most excitatory synapses are formed. It has been postulated that spine dynamics might reflect synaptic plasticity of cortical neurons. To test this hypothesis, we have investigated spine dynamics during the critical period in mouse visual cortex in vivo with and without sensory deprivation. The motility of spines on apical dendrites of layer 5 neurons was assayed by time-lapse two-photon microscopy. Spines were motile at the ages examined, postnatal days (P)21-P42, although motility decreased between P21 and P28 and then remained stable through P42. Binocular deprivation from before the time of eye-opening up-regulated spine motility during the peak of the critical period (P28), without affecting average spine length, class distribution, or density. Deprivation at the start of the critical period had no effect on spine motility, whereas continued deprivation through the end of the critical period appeared to reduce spine motility slightly. We conclude that spine motility might be involved in critical-period plasticity and that reduction of activity during the critical period enhances spine dynamics.
Figures


Similar articles
-
Pyramidal Neurons in Different Cortical Layers Exhibit Distinct Dynamics and Plasticity of Apical Dendritic Spines.Front Neural Circuits. 2017 Jun 19;11:43. doi: 10.3389/fncir.2017.00043. eCollection 2017. Front Neural Circuits. 2017. PMID: 28674487 Free PMC article.
-
Monocular deprivation induces dendritic spine elimination in the developing mouse visual cortex.Sci Rep. 2017 Jul 10;7(1):4977. doi: 10.1038/s41598-017-05337-6. Sci Rep. 2017. PMID: 28694464 Free PMC article.
-
Remodeling of synaptic structure in sensory cortical areas in vivo.J Neurosci. 2006 Mar 15;26(11):3021-9. doi: 10.1523/JNEUROSCI.4454-05.2006. J Neurosci. 2006. PMID: 16540580 Free PMC article.
-
Early Sensory Loss Alters the Dendritic Branching and Spine Density of Supragranular Pyramidal Neurons in Rodent Primary Sensory Cortices.Front Neural Circuits. 2019 Sep 25;13:61. doi: 10.3389/fncir.2019.00061. eCollection 2019. Front Neural Circuits. 2019. PMID: 31611778 Free PMC article.
-
Developmental regulation of spine and filopodial motility in primary visual cortex: reduced effects of activity and sensory deprivation.J Neurobiol. 2004 May;59(2):236-46. doi: 10.1002/neu.10306. J Neurobiol. 2004. PMID: 15085540
Cited by
-
Peripheral deafferentation-driven functional somatosensory map shifts are associated with local, not large-scale dendritic structural plasticity.J Neurosci. 2013 May 29;33(22):9474-87. doi: 10.1523/JNEUROSCI.1032-13.2013. J Neurosci. 2013. PMID: 23719814 Free PMC article.
-
Experience-dependent switch in sign and mechanisms for plasticity in layer 4 of primary visual cortex.J Neurosci. 2012 Aug 1;32(31):10562-73. doi: 10.1523/JNEUROSCI.0622-12.2012. J Neurosci. 2012. PMID: 22855806 Free PMC article.
-
Distribution and effects of the muscarinic receptor subtypes in the primary visual cortex.Front Synaptic Neurosci. 2015 Jun 19;7:10. doi: 10.3389/fnsyn.2015.00010. eCollection 2015. Front Synaptic Neurosci. 2015. PMID: 26150786 Free PMC article. Review.
-
Juvenile depletion of microglia reduces orientation but not high spatial frequency selectivity in mouse V1.Sci Rep. 2022 Jul 27;12(1):12779. doi: 10.1038/s41598-022-15503-0. Sci Rep. 2022. PMID: 35896554 Free PMC article.
-
Methamphetamine Learning Induces Persistent and Selective Nonmuscle Myosin II-Dependent Spine Motility in the Basolateral Amygdala.J Neurosci. 2020 Mar 25;40(13):2695-2707. doi: 10.1523/JNEUROSCI.2182-19.2020. Epub 2020 Feb 17. J Neurosci. 2020. PMID: 32066582 Free PMC article.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

