Alpha interferon inhibits human T-cell leukemia virus type 1 assembly by preventing Gag interaction with rafts
- PMID: 14645593
- PMCID: PMC296084
- DOI: 10.1128/jvi.77.24.13389-13395.2003
Alpha interferon inhibits human T-cell leukemia virus type 1 assembly by preventing Gag interaction with rafts
Abstract
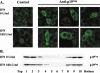
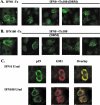
Alpha-2a interferon (IFN-alpha2a) has beneficial clinical effects on human T-cell leukemia virus type 1 (HTLV-1) infection, but its antiviral mechanism of action is unknown. Antiviral effects of IFN-alpha2a were studied in 293T cells expressing HTLV-1 proviral DNA and in HTLV-1-infected cells (HOS/PL, MT2, and HUT102). In 293T cells, an 50% inhibitory concentration of 10 U of IFN-alpha2a/ml was determined by p19 antigen ELISA. Analysis of IFN-treated cells demonstrated no defect in viral protein synthesis but did show a decrease in the level of released virus, as determined by immunoblot assays. Electron microscopy studies of IFN-treated cells revealed neither a defect in the site of virus budding nor tethering of virus particles at the plasma membrane, thus arguing against an effect on virus release. Cell fractionation studies and confocal microscopy showed no effect of IFN on Gag association with membranes. However, the level of Gag association with lipid rafts was decreased, suggesting a role of IFN in inhibiting HTLV-1 assembly.
Figures



Similar articles
-
Role of the human T-cell leukemia virus type 1 PTAP motif in Gag targeting and particle release.J Virol. 2006 Apr;80(7):3634-43. doi: 10.1128/JVI.80.7.3634-3643.2006. J Virol. 2006. PMID: 16537631 Free PMC article.
-
Late assembly motifs of human T-cell leukemia virus type 1 and their relative roles in particle release.J Virol. 2004 Jun;78(12):6636-48. doi: 10.1128/JVI.78.12.6636-6648.2004. J Virol. 2004. PMID: 15163754 Free PMC article.
-
HTLV-1 Gag protein associates with CD82 tetraspanin microdomains at the plasma membrane.Virology. 2006 Mar 1;346(1):194-204. doi: 10.1016/j.virol.2005.10.033. Epub 2005 Dec 1. Virology. 2006. PMID: 16325219
-
Critical Role of the Human T-Cell Leukemia Virus Type 1 Capsid N-Terminal Domain for Gag-Gag Interactions and Virus Particle Assembly.J Virol. 2018 Jun 29;92(14):e00333-18. doi: 10.1128/JVI.00333-18. Print 2018 Jul 15. J Virol. 2018. PMID: 29695435 Free PMC article.
-
Structural Insights into the Mechanism of Human T-cell Leukemia Virus Type 1 Gag Targeting to the Plasma Membrane for Assembly.J Mol Biol. 2021 Sep 17;433(19):167161. doi: 10.1016/j.jmb.2021.167161. Epub 2021 Jul 21. J Mol Biol. 2021. PMID: 34298060 Free PMC article.
Cited by
-
The roles of acquired and innate immunity in human T-cell leukemia virus type 1-mediated diseases.Front Microbiol. 2012 Sep 3;3:323. doi: 10.3389/fmicb.2012.00323. eCollection 2012. Front Microbiol. 2012. PMID: 22969761 Free PMC article.
-
HTLV-1 and innate immunity.Viruses. 2011 Aug;3(8):1374-94. doi: 10.3390/v3081374. Epub 2011 Aug 8. Viruses. 2011. PMID: 21994785 Free PMC article. Review.
-
The potential therapeutic effect of statins in multiple sclerosis: beneficial or detrimental effects.Inflammopharmacology. 2023 Aug;31(4):1671-1682. doi: 10.1007/s10787-023-01240-x. Epub 2023 May 9. Inflammopharmacology. 2023. PMID: 37160526 Review.
-
The Role of Lipids in Retrovirus Replication.Viruses. 2010 May 1;2(5):1146-1180. doi: 10.3390/v2051146. Viruses. 2010. PMID: 20740061 Free PMC article.
-
Retroviral matrix and lipids, the intimate interaction.Retrovirology. 2011 Mar 7;8:15. doi: 10.1186/1742-4690-8-15. Retrovirology. 2011. PMID: 21385335 Free PMC article. Review.
References
-
- Bazarbachi, A., M. E. El-Sabban, R. Nasr, F. Quignon, C. Awaraji, J. Kersual, L. Dianoux, Y. Zermati, J. H. Haidar, O. Hermine, and H. de The. 1999. Arsenic trioxide and interferon-alpha synergize to induce cell cycle arrest and apoptosis in human T-cell lymphotropic virus type I-transformed cells. Blood 93:278-283. - PubMed
-
- Bazarbachi, A., and O. Hermine. 1996. Treatment with a combination of zidovudine and alpha-interferon in naive and pretreated adult T-cell leukemia/lymphoma patients. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 13:S186-S190. - PubMed
-
- Bazarbachi, A., R. Nasr, M. E. El-Sabban, A. Mahe, R. Mahieux, A. Gessain, N. Darwiche, G. Dbaibo, J. Kersual, Y. Zermati, L. Dianoux, M. K. Chelbi-Alix, H. de The, and O. Hermine. 2000. Evidence against a direct cytotoxic effect of alpha interferon and zidovudine in HTLV-I associated adult T cell leukemia/lymphoma. Leukemia 14:716-721. - PubMed
-
- Bouamr, F., L. Garnier, F. Rayne, A. Verna, N. Rebeyrotte, M. Cerutti, and R. Z. Mamoun. 2000. Differential budding efficiencies of human T-cell leukemia virus type I (HTLV-I) Gag and Gag-Pro polyproteins from insect and mammalian cells. Virology 278:597-609. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

