Direct binding of the PDZ domain of Dishevelled to a conserved internal sequence in the C-terminal region of Frizzled
- PMID: 14636582
- PMCID: PMC4381837
- DOI: 10.1016/s1097-2765(03)00427-1
Direct binding of the PDZ domain of Dishevelled to a conserved internal sequence in the C-terminal region of Frizzled
Abstract
The cytoplasmic protein Dishevelled (Dvl) and the associated membrane-bound receptor Frizzled (Fz) are essential in canonical and noncanonical Wnt signaling pathways. However, the molecular mechanisms underlying this signaling are not well understood. By using NMR spectroscopy, we determined that an internal sequence of Fz binds to the conventional peptide binding site in the PDZ domain of Dvl; this type of site typically binds to C-terminal binding motifs. The C-terminal region of the Dvl inhibitor Dapper (Dpr) and Frodo bound to the same site. In Xenopus, Dvl binding peptides of Fz and Dpr/Frodo inhibited canonical Wnt signaling and blocked Wnt-induced secondary axis formation in a dose-dependent manner, but did not block noncanonical Wnt signaling mediated by the DEP domain. Together, our results identify a missing molecular connection within the Wnt pathway. Differences in the binding affinity of the Dvl PDZ domain and its binding partners may be important in regulating signal transduction by Dvl.
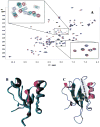
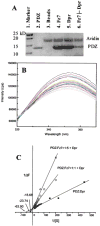
Figures


Similar articles
-
Inhibition of the Wnt signaling pathway by Idax, a novel Dvl-binding protein.Mol Cell Biol. 2001 Jan;21(1):330-42. doi: 10.1128/MCB.21.1.330-342.2001. Mol Cell Biol. 2001. PMID: 11113207 Free PMC article.
-
Wnt/β-catenin signaling requires interaction of the Dishevelled DEP domain and C terminus with a discontinuous motif in Frizzled.Proc Natl Acad Sci U S A. 2012 Apr 3;109(14):E812-20. doi: 10.1073/pnas.1114802109. Epub 2012 Mar 12. Proc Natl Acad Sci U S A. 2012. PMID: 22411803 Free PMC article.
-
Identification of a specific inhibitor of the dishevelled PDZ domain.Biochemistry. 2005 Nov 29;44(47):15495-503. doi: 10.1021/bi0512602. Biochemistry. 2005. PMID: 16300398
-
Dishevelled: at the crossroads of divergent intracellular signaling pathways.Mech Dev. 1999 May;83(1-2):27-37. doi: 10.1016/s0925-4773(99)00046-5. Mech Dev. 1999. PMID: 10507837 Review.
-
Frizzled signalling and cell polarisation in Drosophila and vertebrates.Development. 2003 Oct;130(19):4501-13. doi: 10.1242/dev.00695. Development. 2003. PMID: 12925579 Review.
Cited by
-
Virtual ligand screening combined with NMR to identify Dvl PDZ domain inhibitors targeting the Wnt signaling.Methods Mol Biol. 2012;928:17-28. doi: 10.1007/978-1-62703-008-3_2. Methods Mol Biol. 2012. PMID: 22956130 Free PMC article.
-
Silencing dishevelled-1 sensitizes paclitaxel-resistant human ovarian cancer cells via AKT/GSK-3β/β-catenin signalling.Cell Prolif. 2015 Apr;48(2):249-58. doi: 10.1111/cpr.12161. Epub 2015 Jan 21. Cell Prolif. 2015. PMID: 25643607 Free PMC article.
-
Structure of the C-terminal region of the Frizzled receptor 1 in detergent micelles.Molecules. 2013 Jul 22;18(7):8579-90. doi: 10.3390/molecules18078579. Molecules. 2013. PMID: 23881048 Free PMC article.
-
Dishevelled3 is a novel arginine methyl transferase substrate.Sci Rep. 2012;2:805. doi: 10.1038/srep00805. Epub 2012 Nov 13. Sci Rep. 2012. PMID: 23150776 Free PMC article.
-
Genetics and signaling mechanisms of orofacial clefts.Birth Defects Res. 2020 Nov;112(19):1588-1634. doi: 10.1002/bdr2.1754. Epub 2020 Jul 15. Birth Defects Res. 2020. PMID: 32666711 Free PMC article. Review.
References
-
- Bezprozvanny I, Maximov A. Classification of PDZ domains. FEBS Lett. 2001;509:457–462. - PubMed
-
- Boutros M, Mlodzik M. Dishevelled: at the crossroads of divergent intracellular signaling pathways. Mech Dev. 1999;83:27–37. - PubMed
-
- Boutros M, Paricio N, Strutt DI, Mlodzik M. Dishevelled activates JNK and discriminates between JNK pathways in planar polarity and wingless signaling. Cell. 1998;94:109–118. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

