Latent herpesvirus infection in human trigeminal ganglia causes chronic immune response
- PMID: 14633592
- PMCID: PMC1892378
- DOI: 10.1016/S0002-9440(10)63575-4
Latent herpesvirus infection in human trigeminal ganglia causes chronic immune response
Abstract
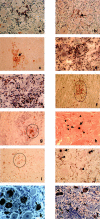
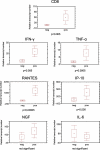
The majority of trigeminal ganglia (TGs) are latently infected with alpha-herpesviruses [herpes simplex virus type-1 (HSV-1) and varicella-zoster virus (VZV)]. Whereas HSV-1 periodically reactivates in the TGs, VZV reactivates very rarely. The goal of this study was to determine whether herpesvirus latency is linked to a local immune cell infiltration in human TGs. T cells positive for the CD3 and CD8 markers, and CD68-positive macrophages were found in 30 of 42 examined TGs from 21 healthy individuals. The presence of immune cells correlated constantly with the occurrence of the HSV-1 latency-associated transcript (LAT) and only irregularly with the presence of latent VZV protein. In contrast, uninfected TGs showed no immune cell infiltration. Quantitative RT-PCR revealed that CD8, interferon-gamma, tumor necrosis factor-alpha, IP-10, and RANTES transcripts were significantly induced in TGs latently infected with HSV-1 but not in uninfected TGs. The persisting lymphocytic cell infiltration and the elevated CD8 and cytokine/chemokine expression in the TGs demonstrate for the first time that latent herpesviral infection in humans is accompanied by a chronic inflammatory process at an immunoprivileged site but without any neuronal destruction. The chronic immune response seems to maintain viral latency and influence viral reactivation.
Figures
Similar articles
-
Latency of alpha-herpes viruses is accompanied by a chronic inflammation in human trigeminal ganglia but not in dorsal root ganglia.J Neuropathol Exp Neurol. 2006 Oct;65(10):1022-30. doi: 10.1097/01.jnen.0000235852.92963.bf. J Neuropathol Exp Neurol. 2006. PMID: 17021407
-
Latency of herpes simplex virus type-1 in human geniculate and vestibular ganglia is associated with infiltration of CD8+ T cells.J Med Virol. 2010 Nov;82(11):1917-20. doi: 10.1002/jmv.21904. J Med Virol. 2010. PMID: 20872719
-
Detection of varicella-zoster virus DNA in 414 human trigeminal ganglia from cadavers by the polymerase chain reaction: a comparison of the detection rate of varicella-zoster virus and herpes simplex virus type 1.J Med Virol. 2010 Feb;82(2):345-9. doi: 10.1002/jmv.21687. J Med Virol. 2010. PMID: 20029810
-
A comparison of herpes simplex virus type 1 and varicella-zoster virus latency and reactivation.J Gen Virol. 2015 Jul;96(Pt 7):1581-602. doi: 10.1099/vir.0.000128. Epub 2015 Mar 20. J Gen Virol. 2015. PMID: 25794504 Free PMC article. Review.
-
Herpesvirus Infections Potentiated by Biologics.Infect Dis Clin North Am. 2020 Jun;34(2):311-339. doi: 10.1016/j.idc.2020.02.006. Infect Dis Clin North Am. 2020. PMID: 32444012 Review.
Cited by
-
Targeting herpetic keratitis by gene therapy.J Ophthalmol. 2012;2012:594869. doi: 10.1155/2012/594869. Epub 2012 Dec 26. J Ophthalmol. 2012. PMID: 23326647 Free PMC article.
-
Bovine herpesvirus 1 regulatory proteins are detected in trigeminal ganglionic neurons during the early stages of stress-induced escape from latency.J Neurovirol. 2015 Oct;21(5):585-91. doi: 10.1007/s13365-015-0339-x. Epub 2015 Apr 10. J Neurovirol. 2015. PMID: 25860382
-
Epidemiology of herpes simplex virus type 1 in the United States: Systematic review, meta-analyses, and meta-regressions.iScience. 2024 Aug 5;27(9):110652. doi: 10.1016/j.isci.2024.110652. eCollection 2024 Sep 20. iScience. 2024. PMID: 39224512 Free PMC article.
-
Illuminating viral infections in the nervous system.Nat Rev Immunol. 2011 May;11(5):318-29. doi: 10.1038/nri2971. Nat Rev Immunol. 2011. PMID: 21508982 Free PMC article. Review.
-
Increased cell-mediated immune responses in patients with recurrent herpes simplex virus type 2 meningitis.Clin Vaccine Immunol. 2011 Apr;18(4):655-60. doi: 10.1128/CVI.00333-10. Epub 2011 Feb 16. Clin Vaccine Immunol. 2011. PMID: 21325490 Free PMC article.
References
-
- Croen DK, Ostrove JM, Dragovic LJ, Smialek JE, Straus SE: Latent herpes simplex virus in human trigeminal ganglia: detection of an immediate early gene ’antisense’ transcript by in situ hybridisation. N Engl J Med 1987, 317:1427-1432 - PubMed
-
- Furuta Y, Takasu T, Sato KC, Fukuda S, Inuyama Y, Nagashima K: Latent herpes virus type 1 in human geniculate ganglia. Acta Neuropathol (Berl) 1992, 84:39-44 - PubMed
-
- Simmons A, Tscharke D, Speck P: The role of immune mechanisms in control of herpes simplex virus infection of the peripheral nervous system. Curr Top Microbiol Immunol 1992, 179:31-56 - PubMed
-
- Mester JC, Rouse BT: The mouse model and understanding immunity to herpes simplex virus. Rev Infect Dis 1991, 13:935-945 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

