Apical recycling systems regulate directional budding of respiratory syncytial virus from polarized epithelial cells
- PMID: 14630951
- PMCID: PMC299925
- DOI: 10.1073/pnas.2434327100
Apical recycling systems regulate directional budding of respiratory syncytial virus from polarized epithelial cells
Abstract
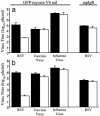
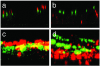
Respiratory syncytial virus (RSV) is the major viral cause of serious lower respiratory tract illness in infants and young children worldwide. RSV infection is limited to the superficial layers of the respiratory epithelium in immunocompetent individuals. Consistent with this in vivo observation, we and others have found that RSV buds preferentially from the apical surface of infected polarized epithelial cells. In contrast, directional budding is not observed in nonpolarized human epithelial cells. These findings suggest that RSV uses specific cellular trafficking pathways to accomplish viral replication. The host cell proteins that regulate directional budding of RSV are undefined. Apical sorting of cellular proteins in polarized epithelial cells involves the apical recycling endosome (ARE). To investigate whether ARE-mediated protein sorting plays a role during RSV replication, we expressed a fragment of the myosin Vb tail that functions as a dominant negative inhibitor of ARE-mediated protein sorting in polarized Madin-Darby canine kidney cells. When these cells were infected with RSV, a >9,000-fold reduction in viral yield was observed. A similar effect on virus replication was observed when a carboxyl-terminal fragment of another ARE-associated protein, the Rab11 family interacting protein 1, was expressed in Madin-Darby canine kidney cells. These data suggest that RSV requires proper ARE-mediated protein sorting for efficient egress from the apical surface of polarized epithelial cells.
Figures
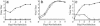


Similar articles
-
Mumps Virus Is Released from the Apical Surface of Polarized Epithelial Cells, and the Release Is Facilitated by a Rab11-Mediated Transport System.J Virol. 2015 Dec;89(23):12026-34. doi: 10.1128/JVI.02048-15. Epub 2015 Sep 16. J Virol. 2015. PMID: 26378159 Free PMC article.
-
Myosin vb is associated with plasma membrane recycling systems.Mol Biol Cell. 2001 Jun;12(6):1843-57. doi: 10.1091/mbc.12.6.1843. Mol Biol Cell. 2001. PMID: 11408590 Free PMC article.
-
The transmembrane domain of the respiratory syncytial virus F protein is an orientation-independent apical plasma membrane sorting sequence.J Virol. 2005 Oct;79(19):12528-35. doi: 10.1128/JVI.79.19.12528-12535.2005. J Virol. 2005. PMID: 16160180 Free PMC article.
-
Respiratory syncytial virus interaction with human airway epithelium.Trends Microbiol. 2013 May;21(5):238-44. doi: 10.1016/j.tim.2013.02.004. Epub 2013 Mar 22. Trends Microbiol. 2013. PMID: 23523320 Review.
-
Respiratory syncytial virus infection and the tight junctions of nasal epithelial cells.Adv Otorhinolaryngol. 2011;72:153-6. doi: 10.1159/000324777. Epub 2011 Aug 18. Adv Otorhinolaryngol. 2011. PMID: 21865717 Review.
Cited by
-
The fusion protein of respiratory syncytial virus triggers p53-dependent apoptosis.J Virol. 2008 Apr;82(7):3236-49. doi: 10.1128/JVI.01887-07. Epub 2008 Jan 23. J Virol. 2008. PMID: 18216092 Free PMC article.
-
Apical transport of influenza A virus ribonucleoprotein requires Rab11-positive recycling endosome.PLoS One. 2011;6(6):e21123. doi: 10.1371/journal.pone.0021123. Epub 2011 Jun 22. PLoS One. 2011. PMID: 21731653 Free PMC article.
-
Viral interactions with host cell Rab GTPases.Small GTPases. 2018 Mar 4;9(1-2):192-201. doi: 10.1080/21541248.2017.1346552. Epub 2017 Sep 18. Small GTPases. 2018. PMID: 28696820 Free PMC article. Review.
-
Mutations in the Transmembrane Domain and Cytoplasmic Tail of Hendra Virus Fusion Protein Disrupt Virus-Like-Particle Assembly.J Virol. 2017 Jun 26;91(14):e00152-17. doi: 10.1128/JVI.00152-17. Print 2017 Jul 15. J Virol. 2017. PMID: 28468881 Free PMC article.
-
Respiratory Syncytial Virus and Cellular Stress Responses: Impact on Replication and Physiopathology.Viruses. 2016 May 12;8(5):124. doi: 10.3390/v8050124. Viruses. 2016. PMID: 27187445 Free PMC article. Review.
References
-
- Compans, R. W. (1995) Curr. Top. Microbiol. Immunol. 202, 209-219. - PubMed
-
- Moll, M., Klenk, H. D., Herrler, G. & Maisner, A. (2001) J. Biol. Chem. 276, 17887-17894. - PubMed
-
- Roth, M. G., Compans, R. W., Giusti, L., Davis, A. R., Nayak, D. P., Gething, M. J. & Sambrook, J. (1983) Cell 33, 435-443. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 DK048370/DK/NIDDK NIH HHS/United States
- P60 DK020593/DK/NIDDK NIH HHS/United States
- T32 CA009385/CA/NCI NIH HHS/United States
- DK43405/DK/NIDDK NIH HHS/United States
- DK58404/DK/NIDDK NIH HHS/United States
- R01 DK043405/DK/NIDDK NIH HHS/United States
- P30 DK058404/DK/NIDDK NIH HHS/United States
- N01AI65298/AI/NIAID NIH HHS/United States
- DK48370/DK/NIDDK NIH HHS/United States
- N01 AI-65298/AI/NIAID NIH HHS/United States
- P30 DK020593/DK/NIDDK NIH HHS/United States
- P30 CA068485/CA/NCI NIH HHS/United States
- CA68485/CA/NCI NIH HHS/United States
- DK20593/DK/NIDDK NIH HHS/United States
- T32 CA09385/CA/NCI NIH HHS/United States
- R21 DE-014039/DE/NIDCR NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

