Regulation of exocytosis from single visualized GABAergic boutons in hippocampal slices
- PMID: 14627631
- PMCID: PMC6740916
- DOI: 10.1523/JNEUROSCI.23-33-10475.2003
Regulation of exocytosis from single visualized GABAergic boutons in hippocampal slices
Abstract
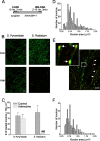
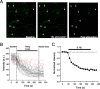
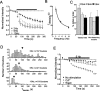
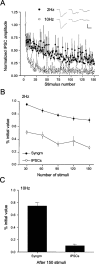
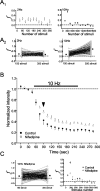
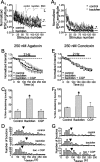
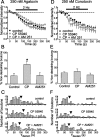
Regulation of GABA release is crucial for normal brain functioning, and GABAA-mediated IPSCs are strongly influenced by repetitive stimulation and neuromodulation. However, GABA exocytosis has not been examined directly in organized tissue. Important issues remain outside the realm of electrophysiological techniques or are complicated by postsynaptic factors. For example, it is not known whether all presynaptic modulators affect release from all boutons in the same way, or whether modulator effects depend on the presence of certain types of voltage-gated calcium channels (VGCCs). To address such issues, we used confocal imaging and styryl dyes to monitor exocytosis from identified GABAergic boutons in organotypic hippocampal slice cultures. Repetitively evoked IPSCs declined more rapidly and completely than exocytosis, suggesting that depletion of filled vesicles cannot fully account for IPSC depression and underscoring the usefulness of directly imaging exocytosis. Stimulation at 10 Hz produced a transient facilitation of exocytosis that was dependent on L-type VGCCs. Using specific toxins, we found that release mediated via N-type and P-type VGCCs had similar properties. Neither baclofen nor a cannabinoid receptor agonist, CP55940, affected all boutons uniformly; they slowed release from some but completely prevented detectable release from others. Increasing stimulus frequency overcame this blockade of release. However, baclofen and CP55940 did not act identically, because only baclofen reduced facilitation and affected bouton releasing via P/Q-type VGCCs. Direct observation thus revealed novel features of GABAergic exocytosis and its regulation that would have been difficult or impossible to detect electrophysiologically. These features advance the understanding of the regulation of synapses and networks by presynaptic inhibition.
Figures

Similar articles
-
GABA B receptor modulation of excitatory and inhibitory synaptic transmission onto rat CA3 hippocampal interneurons.J Physiol. 2003 Jan 15;546(Pt 2):439-53. doi: 10.1113/jphysiol.2002.034017. J Physiol. 2003. PMID: 12527730 Free PMC article.
-
Tonic and synaptically evoked presynaptic inhibition of sensory input to the rat olfactory bulb via GABA(B) heteroreceptors.J Neurophysiol. 2000 Sep;84(3):1194-203. doi: 10.1152/jn.2000.84.3.1194. J Neurophysiol. 2000. PMID: 10979995
-
Syntaxin 1A occludes GABA(B) receptor-induced inhibition of exocytosis downstream of Ca(2+) entry in mouse hippocampal neurons.Neurosci Lett. 2007 Mar 26;415(2):130-4. doi: 10.1016/j.neulet.2007.01.062. Epub 2007 Feb 1. Neurosci Lett. 2007. PMID: 17303333
-
Calcium channel subtypes on single GABAergic presynaptic terminal projecting to rat hippocampal neurons.Brain Res. 2002 Sep 27;951(1):121-9. doi: 10.1016/s0006-8993(02)03148-7. Brain Res. 2002. PMID: 12231465
-
Inhibition [corrected] of olfactory receptor neuron input to olfactory bulb glomeruli mediated by suppression of presynaptic calcium influx.J Neurophysiol. 2005 Oct;94(4):2700-12. doi: 10.1152/jn.00286.2005. Epub 2005 May 25. J Neurophysiol. 2005. PMID: 15917320 Free PMC article.
Cited by
-
Advanced microscopic imaging methods to investigate cortical development and the etiology of mental retardation.Ment Retard Dev Disabil Res Rev. 2005;11(4):303-16. doi: 10.1002/mrdd.20088. Ment Retard Dev Disabil Res Rev. 2005. PMID: 16240412 Free PMC article. Review.
-
Presynaptic AMPA and kainate receptors increase the size of GABAergic terminals and enhance GABA release.Neuropharmacology. 2007 Jun;52(8):1631-40. doi: 10.1016/j.neuropharm.2007.03.010. Epub 2007 Mar 24. Neuropharmacology. 2007. PMID: 17493642 Free PMC article.
-
Vesicle pool heterogeneity at hippocampal glutamate and GABA synapses.J Neurosci. 2007 Sep 12;27(37):9846-54. doi: 10.1523/JNEUROSCI.2803-07.2007. J Neurosci. 2007. PMID: 17855599 Free PMC article.
-
Immunization against GAD induces antibody binding to GAD-independent antigens and brainstem GABAergic neuronal loss.PLoS One. 2013 Sep 18;8(9):e72921. doi: 10.1371/journal.pone.0072921. eCollection 2013. PLoS One. 2013. PMID: 24058450 Free PMC article.
-
NMDA receptors increase the size of GABAergic terminals and enhance GABA release.J Neurosci. 2005 Feb 23;25(8):2024-31. doi: 10.1523/JNEUROSCI.4980-04.2005. J Neurosci. 2005. PMID: 15728842 Free PMC article.
References
-
- Akaike N, Murakami M, Katsurabayashi S, Jin Y-H, Imazawa T ( 2002) Focal stimulation of single GABAergic presynaptic boutons on the rat hippocampal neuron. Neurosci Res 42: 187-195. - PubMed
-
- Alger BE ( 2002) Retrograde signaling in the regulation of synaptic transmission: focus on endocannabinoids. Prog Neurobiol 68: 247-286. - PubMed
-
- Alger BE, Nicoll RA ( 1979) GABA-mediated biphasic inhibitory responses in hippocampus. Nature 281: 315-317. - PubMed
-
- Aravanis AM, Pyle JL, Tsien RW ( 2003) Single synaptic vesicles fusing transiently and successively without loss of identity. Nature 423: 643-647. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
