The isoniazid-NAD adduct is a slow, tight-binding inhibitor of InhA, the Mycobacterium tuberculosis enoyl reductase: adduct affinity and drug resistance
- PMID: 14623976
- PMCID: PMC283515
- DOI: 10.1073/pnas.2235848100
The isoniazid-NAD adduct is a slow, tight-binding inhibitor of InhA, the Mycobacterium tuberculosis enoyl reductase: adduct affinity and drug resistance
Abstract
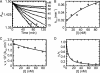
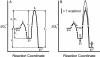
Isoniazid (INH), a frontline antitubercular drug, inhibits InhA, the enoyl reductase from Mycobacterium tuberculosis, by forming a covalent adduct with the NAD cofactor. Here, we report that the INH-NAD adduct is a slow, tight-binding competitive inhibitor of InhA. Demonstration that the adduct binds to WT InhA by a two-step enzyme inhibition mechanism, with initial, weak binding (K(-1) = 16 +/- 11 nM) followed by slow conversion to a final inhibited complex (EI*) with overall Ki = 0.75 +/- 0.08 nM, reconciles existing contradictory values for the inhibitory potency of INH-NAD for InhA. The first order rate constant for conversion of the initial EI complex to EI* (k2 = 0.13 +/- 0.01 min(-1)) is similar to the maximum rate constant observed for InhA inhibition in reaction mixtures containing InhA, INH, NADH, and the INH-activating enzyme KatG (catalase/peroxidase from M. tuberculosis), consistent with an inhibition mechanism in which the adduct forms in solution rather than on the enzyme. Importantly, three mutations that correlate with INH resistance, I21V, I47T, and S94A, have little impact on the inhibition constants. Thus, drug resistance does not result simply from a reduction in affinity of INH-NAD for pure InhA. Instead, we hypothesize that protein-protein interactions within the FASII complex are critical to the mechanism of INH action. Finally, for M161V, an InhA mutation that correlates with resistance to the common biocide triclosan in Mycobacterium smegmatis, binding to form the initial EI complex is significantly weakened, explaining why this mutant inactivates more slowly than WT InhA when incubated with INH, NADH, and KatG.
Figures
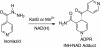

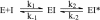
Similar articles
-
Crystallographic studies on the binding of isonicotinyl-NAD adduct to wild-type and isoniazid resistant 2-trans-enoyl-ACP (CoA) reductase from Mycobacterium tuberculosis.J Struct Biol. 2007 Sep;159(3):369-80. doi: 10.1016/j.jsb.2007.04.009. Epub 2007 May 3. J Struct Biol. 2007. PMID: 17588773
-
Crystallographic and pre-steady-state kinetics studies on binding of NADH to wild-type and isoniazid-resistant enoyl-ACP(CoA) reductase enzymes from Mycobacterium tuberculosis.J Mol Biol. 2006 Jun 9;359(3):646-66. doi: 10.1016/j.jmb.2006.03.055. Epub 2006 Apr 21. J Mol Biol. 2006. PMID: 16647717
-
Probing mechanisms of resistance to the tuberculosis drug isoniazid: Conformational changes caused by inhibition of InhA, the enoyl reductase from Mycobacterium tuberculosis.Protein Sci. 2007 Aug;16(8):1617-27. doi: 10.1110/ps.062749007. Epub 2007 Jun 28. Protein Sci. 2007. PMID: 17600151 Free PMC article.
-
Recent Advances and Structural Features of Enoyl-ACP Reductase Inhibitors of Mycobacterium tuberculosis.Arch Pharm (Weinheim). 2016 Nov;349(11):817-826. doi: 10.1002/ardp.201600186. Epub 2016 Oct 24. Arch Pharm (Weinheim). 2016. PMID: 27775177 Review.
-
Recent progress in the identification and development of InhA direct inhibitors of Mycobacterium tuberculosis.Mini Rev Med Chem. 2010 Mar;10(3):181-92. doi: 10.2174/138955710791185064. Mini Rev Med Chem. 2010. PMID: 20408801 Review.
Cited by
-
Protein-protein interaction networks suggest different targets have different propensities for triggering drug resistance.Syst Synth Biol. 2010 Dec;4(4):311-22. doi: 10.1007/s11693-011-9076-5. Epub 2011 Feb 20. Syst Synth Biol. 2010. PMID: 22132058 Free PMC article.
-
Trehalose catalytic shift is an intrinsic factor in Mycobacterium tuberculosis that enhances phenotypic heterogeneity and multidrug resistance.Res Sq [Preprint]. 2024 Sep 13:rs.3.rs-4999164. doi: 10.21203/rs.3.rs-4999164/v1. Res Sq. 2024. PMID: 39315249 Free PMC article. Preprint.
-
Bacterial Oxidases of the Cytochrome bd Family: Redox Enzymes of Unique Structure, Function, and Utility As Drug Targets.Antioxid Redox Signal. 2021 Jun 1;34(16):1280-1318. doi: 10.1089/ars.2020.8039. Epub 2020 Nov 9. Antioxid Redox Signal. 2021. PMID: 32924537 Free PMC article. Review.
-
Antibiotics and resistance: the two-sided coin of the mycobacterial cell wall.Cell Surf. 2020 Sep 2;6:100044. doi: 10.1016/j.tcsw.2020.100044. eCollection 2020 Dec. Cell Surf. 2020. PMID: 32995684 Free PMC article. Review.
-
Identification of potential inhibitor targeting enoyl-acyl carrier protein reductase (InhA) in Mycobacterium tuberculosis: a computational approach.3 Biotech. 2014 Jun;4(3):253-261. doi: 10.1007/s13205-013-0146-0. Epub 2013 Jun 18. 3 Biotech. 2014. PMID: 28324429 Free PMC article.
References
-
- Bloom, B. R. & Murray, C. J. (1992) Science 257, 1055–1064. - PubMed
-
- Heym, B., Honore, N., Truffot-Pernot, C., Banerjee, A., Schurra, C., Jacobs WR, Jr., van Embden, J. D., Grosset, J. H. & Cole, S. T. (1994) Lancet 344, 293–298. - PubMed
-
- Perlman, D. C., ElSadr, W. M., Heifets, L. B., Nelson, E. T., Matts, J. P., Chirgwin, K., Salomon, N., Telzak, E. E., Klein, O., Kreiswirth, B. N., et al. (1997) AIDS 11, 1473–1478. - PubMed
-
- Zhang, Y., Heym, B., Allen, B., Young, D. & Cole, S. (1992) Nature 358, 591–593. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

