Distinct roles for the AAA ATPases NSF and p97 in the secretory pathway
- PMID: 14617820
- PMCID: PMC329284
- DOI: 10.1091/mbc.e03-02-0097
Distinct roles for the AAA ATPases NSF and p97 in the secretory pathway
Abstract
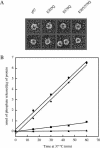
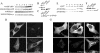
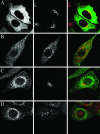
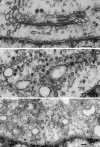
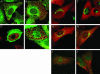
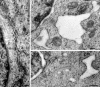
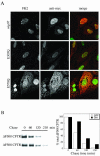
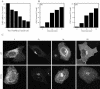
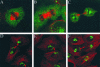
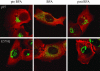
NSF and p97 are related AAA proteins implicated in membrane trafficking and organelle biogenesis. p97 is also involved in pathways that lead to ubiquitin-dependent proteolysis, including ER-associated degradation (ERAD). In this study, we have used dominant interfering ATP-hydrolysis deficient mutants (NSF(E329Q) and p97(E578Q)) to compare the function of these AAA proteins in the secretory pathway of mammalian cells. Expressing NSF(E329Q) promotes disassembly of Golgi stacks into dispersed vesicular structures. It also rapidly inhibits glycosaminoglycan sulfation, reflecting disruption of intra-Golgi transport. In contrast, expressing p97(E578Q) does not affect Golgi structure or function; glycosaminoglycans are normally sulfated and secreted, as is the VSV-G ts045 protein. Instead, expression of p97(E578Q) causes ubiquitinated proteins to accumulate on ER membranes and slows degradation of the ERAD substrate cystic-fibrosis transmembrane-conductance regulator. In addition, expression of p97(E578Q) eventually causes the ER to swell. More specific assessment of effects of p97(E578Q) on organelle assembly shows that the Golgi apparatus disperses and reassembles normally after treatment with brefeldin A and during mitosis. These findings demonstrate that ATP-hydrolysis-dependent activities of NSF and p97 in the cell are not equivalent and suggest that only NSF is directly involved in regulating membrane fusion.
Figures
Similar articles
-
p97/p47-Mediated biogenesis of Golgi and ER.J Biochem. 2005 Feb;137(2):115-9. doi: 10.1093/jb/mvi028. J Biochem. 2005. PMID: 15749824 Review.
-
Unconventional p97/VCP-Mediated Endoplasmic Reticulum-to-Endosome Trafficking of a Retroviral Protein.J Virol. 2021 Jun 24;95(14):e0053121. doi: 10.1128/JVI.00531-21. Epub 2021 Jun 24. J Virol. 2021. PMID: 33952644 Free PMC article.
-
Syntaxin 5 is a common component of the NSF- and p97-mediated reassembly pathways of Golgi cisternae from mitotic Golgi fragments in vitro.Cell. 1998 Mar 6;92(5):603-10. doi: 10.1016/s0092-8674(00)81128-9. Cell. 1998. PMID: 9506515
-
p37 is a p97 adaptor required for Golgi and ER biogenesis in interphase and at the end of mitosis.Dev Cell. 2006 Dec;11(6):803-16. doi: 10.1016/j.devcel.2006.10.016. Dev Cell. 2006. PMID: 17141156
-
Golgi reassembly after mitosis: the AAA family meets the ubiquitin family.Biochim Biophys Acta. 2005 Jun 30;1744(2):108-19. doi: 10.1016/j.bbamcr.2005.03.011. Epub 2005 Apr 9. Biochim Biophys Acta. 2005. Corrected and republished in: Biochim Biophys Acta. 2005 Jul 10;1744(3):481-92. PMID: 15878210 Corrected and republished. Review.
Cited by
-
Loss of soluble N-ethylmaleimide-sensitive factor attachment protein α (αSNAP) induces epithelial cell apoptosis via down-regulation of Bcl-2 expression and disruption of the Golgi.J Biol Chem. 2012 Feb 17;287(8):5928-41. doi: 10.1074/jbc.M111.278358. Epub 2011 Dec 22. J Biol Chem. 2012. PMID: 22194596 Free PMC article.
-
Dissection of Functional Domains of Orc1-2, the Archaeal Global DNA Damage-Responsive Regulator.Int J Mol Sci. 2022 Nov 23;23(23):14609. doi: 10.3390/ijms232314609. Int J Mol Sci. 2022. PMID: 36498936 Free PMC article.
-
Regulation of OPA1 processing and mitochondrial fusion by m-AAA protease isoenzymes and OMA1.J Cell Biol. 2009 Dec 28;187(7):1023-36. doi: 10.1083/jcb.200906084. J Cell Biol. 2009. PMID: 20038678 Free PMC article.
-
The molecular principles governing the activity and functional diversity of AAA+ proteins.Nat Rev Mol Cell Biol. 2020 Jan;21(1):43-58. doi: 10.1038/s41580-019-0183-6. Epub 2019 Nov 21. Nat Rev Mol Cell Biol. 2020. PMID: 31754261 Free PMC article. Review.
-
The allosteric role of the AAA+ domain of ChlD protein from the magnesium chelatase of synechocystis species PCC 6803.J Biol Chem. 2013 Oct 4;288(40):28727-32. doi: 10.1074/jbc.M113.477943. Epub 2013 Aug 12. J Biol Chem. 2013. PMID: 23940041 Free PMC article.
References
-
- Acharya, U., Jacobs, R., Peters, J., Watson, N., Farquhar, M., and Malhotra, V. (1995). The formation of Golgi stacks from vesiculated Golgi membranes requires two distinct fusion events. Cell 82, 895-904. - PubMed
-
- Alvarez, C., and Sztul, E. (1999). Brefeldin A (BFA) disrupts the organization of the microtubule and actin cytoskeletons. Eur. J. Cell Biol. 78, 1-14. - PubMed
-
- Bays, N., and Hampton, R. (2002). Cdc48-Ufd1-Npl 4, stuck in the middle with Ub. Curr. Biol. 12, R366-R371. - PubMed
-
- Coppolino, M.G., Kong, C., Mohtashami, M., Schreiber, A.D., Brumell, J.H., Finlay, B.B., Grinstein, S., Trimble, W.S. (2001). Requirement for N-ethylmaleimide-sensitive factor activity at different stages of bacterial invasion and phagocytosis. J. Biol. Chem. 276, 4772-4780. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

