In vitro assay for neutralizing antibody to hepatitis C virus: evidence for broadly conserved neutralization epitopes
- PMID: 14617769
- PMCID: PMC283569
- DOI: 10.1073/pnas.2335981100
In vitro assay for neutralizing antibody to hepatitis C virus: evidence for broadly conserved neutralization epitopes
Abstract
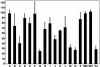
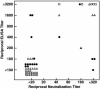
Our understanding of the humoral immune response to hepatitis C virus (HCV) is limited because the virus can be studied only in humans and chimpanzees and because previously described neutralization assays have not been robust or simple to perform. Nevertheless, epidemiologic and laboratory studies suggested that neutralizing Ab to HCV might be important in preventing infection. We have recently described a neutralization assay based on the neutralization of pseudotyped murine retrovirus constructs bearing HCV envelope glycoproteins on their surface. We have applied the assay to well characterized clinical samples from HCV-infected patients and chimpanzees, confirmed the existence of neutralizing Ab to HCV, and validated most previously reported neutralizations of the virus. We did not find neutralizing anti-HCV in resolving infections but did find relatively high titers (>1:320) of such Ab in chronic infections. Neutralizing Ab was directed not only to epitope(s) in the hypervariable region of the E2 envelope protein but also to one or more epitopes elsewhere in the envelope of the virus. Neutralizing Ab was broadly reactive and could neutralize pseudotype particles bearing the envelope glycoproteins of two different subgenotypes (1a and 1b). The ability to assay neutralizing anti-HCV should permit an assessment of the prospects for successful Ab-mediated passive and active immunoprophylaxis against hepatitis C.
Figures
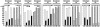

Similar articles
-
Murine antibodies against E2 and hypervariable region 1 cross-reactively capture hepatitis C virus.Virology. 1998 Nov 10;251(1):158-64. doi: 10.1006/viro.1998.9393. Virology. 1998. PMID: 9813211
-
Evidence for cross-genotype neutralization of hepatitis C virus pseudo-particles and enhancement of infectivity by apolipoprotein C1.Proc Natl Acad Sci U S A. 2005 Mar 22;102(12):4560-5. doi: 10.1073/pnas.0501275102. Epub 2005 Mar 14. Proc Natl Acad Sci U S A. 2005. PMID: 15767578 Free PMC article.
-
Antibodies directed to envelope proteins of hepatitis C virus outside of hypervariable region 1.Virology. 1998 Apr 10;243(2):313-21. doi: 10.1006/viro.1998.9069. Virology. 1998. PMID: 9568031
-
Virus-neutralizing antibodies to hepatitis C virus.J Viral Hepat. 2013 Jun;20(6):369-76. doi: 10.1111/jvh.12094. Epub 2013 Apr 4. J Viral Hepat. 2013. PMID: 23647953 Review.
-
[Neutralizing antibodies in hepatitis C virus infection].Gastroenterol Clin Biol. 2008 May;32(5 Pt 1):491-8. doi: 10.1016/j.gcb.2008.02.026. Epub 2008 May 7. Gastroenterol Clin Biol. 2008. PMID: 18467058 Review. French.
Cited by
-
Adaptive Immune Response against Hepatitis C Virus.Int J Mol Sci. 2020 Aug 6;21(16):5644. doi: 10.3390/ijms21165644. Int J Mol Sci. 2020. PMID: 32781731 Free PMC article. Review.
-
Overcoming hurdles in hepatitis C virus research: efficient production of infectious virus in cell culture.Int J Biomed Sci. 2008 Jun;4(2):82-8. Int J Biomed Sci. 2008. PMID: 23675072 Free PMC article.
-
Naturally occurring antibodies that recognize linear epitopes in the amino terminus of the hepatitis C virus E2 protein confer noninterfering, additive neutralization.J Virol. 2012 Mar;86(5):2739-49. doi: 10.1128/JVI.06492-11. Epub 2011 Dec 14. J Virol. 2012. PMID: 22171278 Free PMC article.
-
Characterization of the envelope glycoproteins associated with infectious hepatitis C virus.J Virol. 2010 Oct;84(19):10159-68. doi: 10.1128/JVI.01180-10. Epub 2010 Jul 28. J Virol. 2010. PMID: 20668082 Free PMC article.
-
Sequence diversity of hepatitis C virus: implications for immune control and therapy.World J Gastroenterol. 2007 Sep 28;13(36):4808-17. doi: 10.3748/wjg.v13.i36.4808. World J Gastroenterol. 2007. PMID: 17828811 Free PMC article. Review.
References
-
- Shimizu, Y. K., Igarashi, H., Kiyohara, T., Cabezon, T., Farci, P., Purcell, R. H. & Yoshikura, H. (1996) Virology 223, 409–412. - PubMed
-
- Yu, M. W., Shen, L., Major, M. E., Feinstone, S. M. & Jong, J. S. (2002) in Viral Hepatitis and Liver Disease, eds. Margolis, H. S., Alter, M. J., Liang, T. J. & Dienstag, J. L. (International Medical Press, Atlanta), pp. 374–377.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

