Coding of predicted reward omission by dopamine neurons in a conditioned inhibition paradigm
- PMID: 14614099
- PMCID: PMC6741002
- DOI: 10.1523/JNEUROSCI.23-32-10402.2003
Coding of predicted reward omission by dopamine neurons in a conditioned inhibition paradigm
Abstract
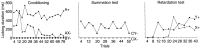
Animals learn not only about stimuli that predict reward but also about those that signal the omission of an expected reward. We used a conditioned inhibition paradigm derived from animal learning theory to train a discrimination between a visual stimulus that predicted reward (conditioned excitor) and a second stimulus that predicted the omission of reward (conditioned inhibitor). Performing the discrimination required attention to both the conditioned excitor and the inhibitor; however, dopamine neurons showed very different responses to the two classes of stimuli. Conditioned inhibitors elicited considerable depressions in 48 of 69 neurons (median of 35% below baseline) and minor activations in 29 of 69 neurons (69% above baseline), whereas reward-predicting excitors induced pure activations in all 69 neurons tested (242% above baseline), thereby demonstrating that the neurons discriminated between conditioned stimuli predicting reward versus nonreward. The discriminative responses to stimuli with differential reward-predicting but common attentional functions indicate differential neural coding of reward prediction and attention. The neuronal responses appear to reflect reward prediction errors, thus suggesting an extension of the correspondence between learning theory and activity of single dopamine neurons to the prediction of nonreward.
Figures





Similar articles
-
Dopamine responses comply with basic assumptions of formal learning theory.Nature. 2001 Jul 5;412(6842):43-8. doi: 10.1038/35083500. Nature. 2001. PMID: 11452299
-
Involvement of basal ganglia and orbitofrontal cortex in goal-directed behavior.Prog Brain Res. 2000;126:193-215. doi: 10.1016/S0079-6123(00)26015-9. Prog Brain Res. 2000. PMID: 11105648 Review.
-
Neuronal responses of the rat amygdala during extinction and reassociation learning in elementary and configural associative tasks.Eur J Neurosci. 2002 Feb;15(4):753-68. doi: 10.1046/j.1460-9568.2002.01889.x. Eur J Neurosci. 2002. PMID: 11886454
-
Emotional and behavioral correlates of the anterior cingulate cortex during associative learning in rats.Neuroscience. 1999;93(4):1271-87. doi: 10.1016/s0306-4522(99)00216-x. Neuroscience. 1999. PMID: 10501451
-
Predictive reward signal of dopamine neurons.J Neurophysiol. 1998 Jul;80(1):1-27. doi: 10.1152/jn.1998.80.1.1. J Neurophysiol. 1998. PMID: 9658025 Review.
Cited by
-
Conditioned inhibition of fear and reward in male and female rats.Neurobiol Learn Mem. 2024 Feb;208:107881. doi: 10.1016/j.nlm.2023.107881. Epub 2023 Dec 20. Neurobiol Learn Mem. 2024. PMID: 38135111
-
Are you or aren't you? Challenges associated with physiologically identifying dopamine neurons.Trends Neurosci. 2012 Jul;35(7):422-30. doi: 10.1016/j.tins.2012.02.003. Epub 2012 Mar 28. Trends Neurosci. 2012. PMID: 22459161 Free PMC article. Review.
-
Conjunctive encoding of movement and reward by ventral tegmental area neurons in the freely navigating rodent.Behav Neurosci. 2010 Apr;124(2):234-47. doi: 10.1037/a0018865. Behav Neurosci. 2010. PMID: 20364883 Free PMC article.
-
The neurocircuitry of addiction: an overview.Br J Pharmacol. 2008 May;154(2):261-74. doi: 10.1038/bjp.2008.51. Epub 2008 Mar 3. Br J Pharmacol. 2008. PMID: 18311189 Free PMC article. Review.
-
Midbrain dopamine neurons signal preference for advance information about upcoming rewards.Neuron. 2009 Jul 16;63(1):119-26. doi: 10.1016/j.neuron.2009.06.009. Neuron. 2009. PMID: 19607797 Free PMC article.
References
-
- Briand KA, Hening W, Poizner H, Sereno AB ( 2001) Automatic orienting of visuospatial attention in Parkinson's disease. Neuropsychologia 39: 1240-1249. - PubMed
-
- Brown RG, Marsden CD ( 1988) Internal versus external cues and the control of attention in Parkinson's disease. Brain 111: 323-345. - PubMed
-
- Carli M, Evenden JL, Robbins TW ( 1985) Depletion of unilateral striatal dopamine impairs initiation of contralateral actions and not sensory attention. Nature 313: 679-682. - PubMed
-
- Egeth HE, Yantis S ( 1997) Visual attention: control, representation, and time course. Annu Rev Psychol 48: 269-297. - PubMed
-
- Eisenberger R ( 1972) Explanation of rewards that do not reduce tissue needs. Psychol Bull 77: 319-339. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
