Ribosomal protein L11 negatively regulates oncoprotein MDM2 and mediates a p53-dependent ribosomal-stress checkpoint pathway
- PMID: 14612427
- PMCID: PMC262682
- DOI: 10.1128/MCB.23.23.8902-8912.2003
Ribosomal protein L11 negatively regulates oncoprotein MDM2 and mediates a p53-dependent ribosomal-stress checkpoint pathway
Abstract
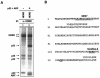
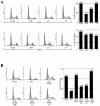
The gene encoding p53 mediates a major tumor suppression pathway that is frequently altered in human cancers. p53 function is kept at a low level during normal cell growth and is activated in response to various cellular stresses. The MDM2 oncoprotein plays a key role in negatively regulating p53 activity by either direct repression of p53 transactivation activity in the nucleus or promotion of p53 degradation in the cytoplasm. DNA damage and oncogenic insults, the two best-characterized p53-dependent checkpoint pathways, both activate p53 through inhibition of MDM2. Here we report that the human homologue of MDM2, HDM2, binds to ribosomal protein L11. L11 binds a central region in HDM2 that is distinct from the ARF binding site. We show that the functional consequence of L11-HDM2 association, like that with ARF, results in the prevention of HDM2-mediated p53 ubiquitination and degradation, subsequently restoring p53-mediated transactivation, accumulating p21 protein levels, and inducing a p53-dependent cell cycle arrest by canceling the inhibitory function of HDM2. Interference with ribosomal biogenesis by a low concentration of actinomycin D is associated with an increased L11-HDM2 interaction and subsequent p53 stabilization. We suggest that L11 functions as a negative regulator of HDM2 and that there might exist in vivo an L11-HDM2-p53 pathway for monitoring ribosomal integrity.
Figures
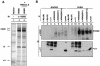



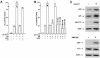


Similar articles
-
Regulation of HDM2 activity by the ribosomal protein L11.Cancer Cell. 2003 Jun;3(6):577-87. doi: 10.1016/s1535-6108(03)00134-x. Cancer Cell. 2003. PMID: 12842086
-
Inhibition of MDM2-mediated p53 ubiquitination and degradation by ribosomal protein L5.J Biol Chem. 2004 Oct 22;279(43):44475-82. doi: 10.1074/jbc.M403722200. Epub 2004 Aug 11. J Biol Chem. 2004. PMID: 15308643
-
Inhibition of HDM2 and activation of p53 by ribosomal protein L23.Mol Cell Biol. 2004 Sep;24(17):7669-80. doi: 10.1128/MCB.24.17.7669-7680.2004. Mol Cell Biol. 2004. PMID: 15314174 Free PMC article.
-
Control of p53 ubiquitination and nuclear export by MDM2 and ARF.Cell Growth Differ. 2001 Apr;12(4):175-86. Cell Growth Differ. 2001. PMID: 11331246 Review.
-
Functions of the MDM2 oncoprotein.Cell Mol Life Sci. 1999 Jan;55(1):96-107. doi: 10.1007/s000180050273. Cell Mol Life Sci. 1999. PMID: 10065155 Free PMC article. Review.
Cited by
-
Suprainduction of p53 by disruption of 40S and 60S ribosome biogenesis leads to the activation of a novel G2/M checkpoint.Genes Dev. 2012 May 15;26(10):1028-40. doi: 10.1101/gad.189951.112. Genes Dev. 2012. PMID: 22588717 Free PMC article.
-
Imatinib mesylate decreases the cytotoxic effect of roscovitine on human glioblastoma cells in vitro and the role of midkine.Oncol Lett. 2012 Jan;3(1):200-208. doi: 10.3892/ol.2011.434. Epub 2011 Oct 4. Oncol Lett. 2012. PMID: 22740881 Free PMC article.
-
Human NOP2/NSUN1 regulates ribosome biogenesis through non-catalytic complex formation with box C/D snoRNPs.Nucleic Acids Res. 2022 Oct 14;50(18):10695-10716. doi: 10.1093/nar/gkac817. Nucleic Acids Res. 2022. PMID: 36161484 Free PMC article.
-
Ribosomal proteins RPL37, RPS15 and RPS20 regulate the Mdm2-p53-MdmX network.PLoS One. 2013 Jul 16;8(7):e68667. doi: 10.1371/journal.pone.0068667. Print 2013. PLoS One. 2013. PMID: 23874713 Free PMC article.
-
MDM2 and MDMX: Alone and together in regulation of p53.Transl Cancer Res. 2012 Aug;1(2):88-89. Transl Cancer Res. 2012. PMID: 23002429 Free PMC article.
References
-
- Bates, S., A. C. Phillips, P. Clark, F. Stott, G. Peters, R. Ludwig, and K. H. Vousden. 1998. p14ARF links the tumor suppressors RB and p53. Nature 395:124-125. - PubMed
-
- Corvi, R., L. Savelyeva, S. Breit, A. Wenzel, R. Handgretinger, J. Barak, M. Oren, L. Amler, and M. Schwab. 1995. Non-syntenic amplification of MDM2 and MYCN in human neuroblastoma. Oncogene 10:1081-1086. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
