Acetylation of androgen receptor enhances coactivator binding and promotes prostate cancer cell growth
- PMID: 14612401
- PMCID: PMC262657
- DOI: 10.1128/MCB.23.23.8563-8575.2003
Acetylation of androgen receptor enhances coactivator binding and promotes prostate cancer cell growth
Abstract
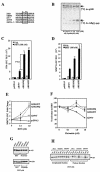
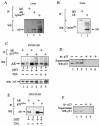
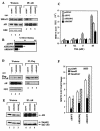
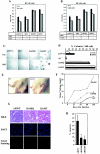
Modification by acetylation occurs at epsilon-amino lysine residues of histones and transcription factors. Unlike phosphorylation, a direct link between transcription factor acetylation and cellular growth or apoptosis has not been established. We show that the nuclear androgen receptor (AR), a DNA-binding transcriptional regulator, is acetylated in vivo. The acetylation of the AR is induced by ligand dihydrotestosterone and by histone deacetylase (HDAC) inhibitors in living cells. Direct AR acetylation augmented p300 binding in vitro. Constructs mimicking neutral polar substitution acetylation (AR(K630Q), AR(K630T)) enhanced p300 binding and reduced N-CoR/HDAC/Smad3 corepressor binding, whereas charged residue substitution (AR(K630R)) reduced p300 binding and enhanced corepressor binding. The AR acetylation mimics promoted cell survival and growth of prostate cancer cells in soft agar and in nude mice and augmented transcription of a subset of growth control target gene promoters. Thus, transcription factor acetylation regulates coactivator/corepressor complex binding, altering expression of specific growth control genes to promote aberrant cellular growth in vivo.
Figures



Similar articles
-
The androgen receptor acetylation site regulates cAMP and AKT but not ERK-induced activity.J Biol Chem. 2004 Jul 9;279(28):29436-49. doi: 10.1074/jbc.M313466200. Epub 2004 Apr 30. J Biol Chem. 2004. PMID: 15123687
-
p300 and p300/cAMP-response element-binding protein-associated factor acetylate the androgen receptor at sites governing hormone-dependent transactivation.J Biol Chem. 2000 Jul 7;275(27):20853-60. doi: 10.1074/jbc.M000660200. J Biol Chem. 2000. PMID: 10779504
-
Activation of p300 histone acetyltransferase activity and acetylation of the androgen receptor by bombesin in prostate cancer cells.Oncogene. 2006 Mar 30;25(14):2011-21. doi: 10.1038/sj.onc.1209231. Oncogene. 2006. PMID: 16434977
-
Acetylation of nuclear receptors in cellular growth and apoptosis.Biochem Pharmacol. 2004 Sep 15;68(6):1199-208. doi: 10.1016/j.bcp.2004.05.037. Biochem Pharmacol. 2004. PMID: 15313417 Review.
-
Expression and function of androgen receptor coactivators in prostate cancer.J Steroid Biochem Mol Biol. 2004 Nov;92(4):265-71. doi: 10.1016/j.jsbmb.2004.10.003. Epub 2004 Dec 19. J Steroid Biochem Mol Biol. 2004. PMID: 15663989 Review.
Cited by
-
Divergent Binding and Transactivation by Two Related Steroid Receptors at the Same Response Element.J Biol Chem. 2016 May 27;291(22):11899-910. doi: 10.1074/jbc.M115.684480. Epub 2016 Apr 7. J Biol Chem. 2016. PMID: 27056330 Free PMC article.
-
The role of intracrine androgen metabolism, androgen receptor and apoptosis in the survival and recurrence of prostate cancer during androgen deprivation therapy.Curr Drug Targets. 2013 Apr;14(4):420-40. doi: 10.2174/1389450111314040004. Curr Drug Targets. 2013. PMID: 23565755 Free PMC article. Review.
-
FT-6876, a Potent and Selective Inhibitor of CBP/p300, is Active in Preclinical Models of Androgen Receptor-Positive Breast Cancer.Target Oncol. 2023 Mar;18(2):269-285. doi: 10.1007/s11523-023-00949-7. Epub 2023 Feb 24. Target Oncol. 2023. PMID: 36826464 Free PMC article.
-
Evidence that HDAC7 acts as an epigenetic "reader" of AR acetylation through NCoR-HDAC3 dissociation.Cell Chem Biol. 2022 Jul 21;29(7):1162-1173.e5. doi: 10.1016/j.chembiol.2022.05.008. Epub 2022 Jun 15. Cell Chem Biol. 2022. PMID: 35709754 Free PMC article.
-
The role of histone deacetylases in prostate cancer.Epigenetics. 2008 Nov;3(6):300-9. doi: 10.4161/epi.3.6.7273. Epub 2008 Nov 24. Epigenetics. 2008. PMID: 19029799 Free PMC article. Review.
References
-
- Abate-Shen, C., and M. M. Shen. 2000. Molecular genetics of prostate cancer. Genes Dev. 14:2410-2434. - PubMed
-
- Alland, L., R. Muhle, H. J. Hou, J. Potes, L. Chin, N. Schreiber-Agus, and R. A. DePinho. 1997. Role for N-CoR and histone deacetylase in Sin3-mediated transcriptional repression. Nature 387:49-55. - PubMed
-
- Chen, D., H. Ma, H. Hong, S. S. Koh, S. M. Huang, B. T. Schurter, D. W. Aswad, and M. R. Stallcup. 1999. Regulation of transcription by a protein methyltransferase. Science 284:2174-2177. - PubMed
-
- Chen, J. D., and R. M. Evans. 1995. A transcriptional co-repressor that interacts with nuclear hormone receptors. Nature 377:454-457. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 CA075503/CA/NCI NIH HHS/United States
- R01CA75503/CA/NCI NIH HHS/United States
- R01-CA65647/CA/NCI NIH HHS/United States
- R21 DK065220/DK/NIDDK NIH HHS/United States
- R03 AG2033/AG/NIA NIH HHS/United States
- P30 CA051008/CA/NCI NIH HHS/United States
- R01 CA070896/CA/NCI NIH HHS/United States
- P30 CA51008-13/CA/NCI NIH HHS/United States
- 1 R21DK065220-01/DK/NIDDK NIH HHS/United States
- R01 CA83979/CA/NCI NIH HHS/United States
- R01CA70896/CA/NCI NIH HHS/United States
- R01CA86072/CA/NCI NIH HHS/United States
- R01 CA086072/CA/NCI NIH HHS/United States
- R01 CA083979/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous
