COX-2 induction during murine gammaherpesvirus 68 infection leads to enhancement of viral gene expression
- PMID: 14610197
- PMCID: PMC262602
- DOI: 10.1128/jvi.77.23.12753-12763.2003
COX-2 induction during murine gammaherpesvirus 68 infection leads to enhancement of viral gene expression
Abstract
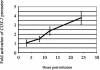
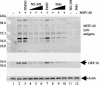
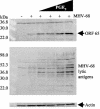
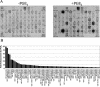
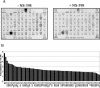
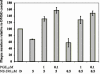
The murine gammaherpesvirus 68 (MHV-68 or gammaHV-68) model provides many advantages for studying virus-host interactions involved in gammaherpesvirus replication, including the role of cellular responses to infection. We examined the effects of cellular cyclooxygenase-2 (COX-2) and its by-product prostaglandin E(2) (PGE(2)) on MHV-68 gene expression and protein production following de novo infection of cultured cells. Western blot analyses revealed an induction of COX-2 protein in MHV-68-infected cells but not in cells infected with UV-irradiated MHV-68. Luciferase reporter assays demonstrated activation of the COX-2 promoter during MHV-68 replication. Two nonsteroidal anti-inflammatory drugs, a COX-2-specific inhibitor (NS-398) and a COX-1-COX-2 inhibitor (indomethacin), substantially reduced MHV-68 protein production in infected cells. Inhibition of viral protein expression and virion production by NS-398 was reversed in the presence of exogenous PGE(2). Global gene expression analysis using an MHV-68 DNA array showed that PGE(2) increased production of multiple viral gene products, and NS-398 inhibited production of many of the same genes. These studies suggest that COX-2 activity and PGE(2) production may play significant roles during MHV-68 de novo infection.
Figures
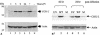


Similar articles
-
Cyclooxygenase 2 induced by Kaposi's sarcoma-associated herpesvirus early during in vitro infection of target cells plays a role in the maintenance of latent viral gene expression.J Virol. 2006 Jul;80(13):6534-52. doi: 10.1128/JVI.00231-06. J Virol. 2006. PMID: 16775340 Free PMC article.
-
Dendritic cells issued in vitro from bone marrow produce PGE(2) that contributes to the immunomodulation induced by antigen-presenting cells.Cell Immunol. 2001 Apr 10;209(1):19-28. doi: 10.1006/cimm.2001.1785. Cell Immunol. 2001. PMID: 11414733
-
Inhibition of angiotensin II activity enhanced the antitumor effect of cyclooxygenase-2 inhibitors via insulin-like growth factor I receptor pathway.Cancer Res. 2003 Oct 15;63(20):6726-34. Cancer Res. 2003. PMID: 14583467
-
Rta of murine gammaherpesvirus 68 reactivates the complete lytic cycle from latency.J Virol. 2000 Apr;74(8):3659-67. doi: 10.1128/jvi.74.8.3659-3667.2000. J Virol. 2000. PMID: 10729142 Free PMC article.
-
Murine gammaherpesvirus 68: a model for the study of gammaherpesvirus pathogenesis.Trends Microbiol. 1998 Jul;6(7):276-82. doi: 10.1016/s0966-842x(98)01306-7. Trends Microbiol. 1998. PMID: 9717216 Review.
Cited by
-
Cyclooxygenase-2-prostaglandin E2-eicosanoid receptor inflammatory axis: a key player in Kaposi's sarcoma-associated herpes virus associated malignancies.Transl Res. 2013 Aug;162(2):77-92. doi: 10.1016/j.trsl.2013.03.004. Epub 2013 Apr 6. Transl Res. 2013. PMID: 23567332 Free PMC article. Review.
-
Proteomics of Bronchoalveolar Lavage Fluid Reveals a Lung Oxidative Stress Response in Murine Herpesvirus-68 Infection.Viruses. 2018 Nov 27;10(12):670. doi: 10.3390/v10120670. Viruses. 2018. PMID: 30486363 Free PMC article.
-
Inhibition of cyclooxygenase activity reduces rotavirus infection at a postbinding step.J Virol. 2004 Sep;78(18):9721-30. doi: 10.1128/JVI.78.18.9721-9730.2004. J Virol. 2004. PMID: 15331705 Free PMC article.
-
Cyclooxygenase 2 induced by Kaposi's sarcoma-associated herpesvirus early during in vitro infection of target cells plays a role in the maintenance of latent viral gene expression.J Virol. 2006 Jul;80(13):6534-52. doi: 10.1128/JVI.00231-06. J Virol. 2006. PMID: 16775340 Free PMC article.
-
Cyclooxygenase-1 and -2 are required for production of infectious pseudorabies virus.J Virol. 2004 Dec;78(23):12964-74. doi: 10.1128/JVI.78.23.12964-12974.2004. J Virol. 2004. PMID: 15542648 Free PMC article.
References
-
- Barnes, A., H. Dyson, N. P. Sunil-Chandra, P. Collins, and A. A. Nash. 1999. 2′-Deoxy-5-ethyl-beta-4′-thiouridine inhibits replication of murine gammaherpesvirus and delays the onset of virus latency. Antivir. Chem. Chemother. 10:321-326. - PubMed
-
- Bratcher, D. F., C. J. Harrison, N. Bourne, L. R. Stanberry, and D. I. Bernstein. 1993. Effect of indomethacin on ultraviolet radiation-induced recurrent herpes simplex virus disease in guinea-pigs. J. Gen. Virol. 74:1951-1954. - PubMed
-
- Brock, T. G., R. W. McNish, and M. Peters-Golden. 1999. Arachidonic acid is preferentially metabolized by cyclooxygenase-2 to prostacyclin and prostaglandin E2. J. Biol. Chem. 274:11660-11666. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

