Strand-specific RNA synthesis defects in a poliovirus with a mutation in protein 3A
- PMID: 14610190
- PMCID: PMC262582
- DOI: 10.1128/jvi.77.23.12679-12691.2003
Strand-specific RNA synthesis defects in a poliovirus with a mutation in protein 3A
Abstract
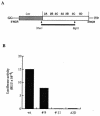
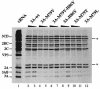
Substitution of a methionine residue at position 79 in poliovirus protein 3A with valine or threonine caused defective viral RNA synthesis, manifested as delayed onset and reduced yield of viral RNA, in HeLa cells transfected with a luciferase-containing replicon. Viruses containing these same mutations produced small or minute plaques that generated revertants upon further passage, with either wild-type 3A sequences or additional nearby compensating mutations. Translation and polyprotein processing were not affected by the mutations, and 3AB proteins containing the altered amino acids at position 79 showed no detectable loss of membrane-binding activity. Analysis of individual steps of viral RNA synthesis in HeLa cell extracts that support translation and replication of viral RNA showed that VPg uridylylation and negative-strand RNA synthesis occurred normally from mutant viral RNA; however, positive-strand RNA synthesis was specifically reduced. The data suggest that a function of viral protein 3A is required for positive-strand RNA synthesis but not for production of negative strands.
Figures






Similar articles
-
Poliovirus CRE-dependent VPg uridylylation is required for positive-strand RNA synthesis but not for negative-strand RNA synthesis.J Virol. 2003 Apr;77(8):4739-50. doi: 10.1128/jvi.77.8.4739-4750.2003. J Virol. 2003. PMID: 12663781 Free PMC article.
-
Genetic and biochemical studies of poliovirus cis-acting replication element cre in relation to VPg uridylylation.J Virol. 2000 Nov;74(22):10371-80. doi: 10.1128/jvi.74.22.10371-10380.2000. J Virol. 2000. PMID: 11044081 Free PMC article.
-
Viability of poliovirus/rhinovirus VPg chimeric viruses and identification of an amino acid residue in the VPg gene critical for viral RNA replication.J Virol. 2003 Jul;77(13):7434-43. doi: 10.1128/jvi.77.13.7434-7443.2003. J Virol. 2003. PMID: 12805442 Free PMC article.
-
Molecular biology and cell-free synthesis of poliovirus.Biologicals. 1993 Dec;21(4):349-56. doi: 10.1006/biol.1993.1095. Biologicals. 1993. PMID: 8024750 Review.
-
Uridylylation of the genome-linked protein of poliovirus in vitro is dependent upon an endogenous RNA template.Virus Res. 1987 Sep;8(3):193-204. doi: 10.1016/0168-1702(87)90015-3. Virus Res. 1987. PMID: 2825442 Review.
Cited by
-
Effects of picornavirus 3A Proteins on Protein Transport and GBF1-dependent COP-I recruitment.J Virol. 2006 Dec;80(23):11852-60. doi: 10.1128/JVI.01225-06. Epub 2006 Sep 27. J Virol. 2006. PMID: 17005635 Free PMC article.
-
Deletion mutants of VPg reveal new cytopathology determinants in a picornavirus.PLoS One. 2010 May 20;5(5):e10735. doi: 10.1371/journal.pone.0010735. PLoS One. 2010. PMID: 20505767 Free PMC article.
-
New small-molecule inhibitors effectively blocking picornavirus replication.J Virol. 2014 Oct;88(19):11091-107. doi: 10.1128/JVI.01877-14. Epub 2014 Jul 9. J Virol. 2014. PMID: 25008939 Free PMC article.
-
Secretory Carrier Membrane Protein 3 Interacts with 3A Viral Protein of Enterovirus and Participates in Viral Replication.Microbiol Spectr. 2021 Sep 3;9(1):e0047521. doi: 10.1128/Spectrum.00475-21. Epub 2021 Aug 11. Microbiol Spectr. 2021. PMID: 34378951 Free PMC article.
-
Testing the modularity of the N-terminal amphipathic helix conserved in picornavirus 2C proteins and hepatitis C NS5A protein.Virology. 2006 Jan 20;344(2):453-67. doi: 10.1016/j.virol.2005.08.044. Epub 2005 Oct 14. Virology. 2006. PMID: 16226781 Free PMC article.
References
-
- Aldabe, R., and L. Carrasco. 1995. Induction of membrane proliferation by poliovirus proteins 2C and 2BC. Biochem. Biophys. Res. Commun. 206:64-76. - PubMed
-
- Banerjee, R., W. Tsai, W. Kim, and A. Dasgupta. 2001. Interaction of poliovirus-encoded 2C/2BC polypeptides with the 3′ terminus negative-strand cloverleaf requires an intact stem-loop b. Virology 280:41-51. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

