The AP-1A and AP-1B clathrin adaptor complexes define biochemically and functionally distinct membrane domains
- PMID: 14581457
- PMCID: PMC2173537
- DOI: 10.1083/jcb.200309020
The AP-1A and AP-1B clathrin adaptor complexes define biochemically and functionally distinct membrane domains
Abstract
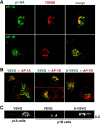
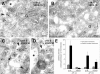
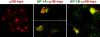
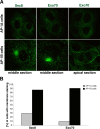
Most epithelial cells contain two AP-1 clathrin adaptor complexes. AP-1A is ubiquitously expressed and involved in transport between the TGN and endosomes. AP-1B is expressed only in epithelia and mediates the polarized targeting of membrane proteins to the basolateral surface. Both AP-1 complexes are heterotetramers and differ only in their 50-kD mu1A or mu1B subunits. Here, we show that AP-1A and AP-1B, together with their respective cargoes, define physically and functionally distinct membrane domains in the perinuclear region. Expression of AP-1B (but not AP-1A) enhanced the recruitment of at least two subunits of the exocyst complex (Sec8 and Exo70) required for basolateral transport. By immunofluorescence and cell fractionation, the exocyst subunits were found to selectively associate with AP-1B-containing membranes that were both distinct from AP-1A-positive TGN elements and more closely apposed to transferrin receptor-positive recycling endosomes. Thus, despite the similarity of the two AP-1 complexes, AP-1A and AP-1B exhibit great specificity for endosomal transport versus cell polarity.
Figures


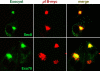
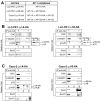


Similar articles
-
Distribution and function of AP-1 clathrin adaptor complexes in polarized epithelial cells.J Cell Biol. 2001 Feb 5;152(3):595-606. doi: 10.1083/jcb.152.3.595. J Cell Biol. 2001. PMID: 11157985 Free PMC article.
-
The clathrin adaptor AP-1A mediates basolateral polarity.Dev Cell. 2012 Apr 17;22(4):811-23. doi: 10.1016/j.devcel.2012.02.004. Dev Cell. 2012. PMID: 22516199 Free PMC article.
-
A novel clathrin adaptor complex mediates basolateral targeting in polarized epithelial cells.Cell. 1999 Oct 15;99(2):189-98. doi: 10.1016/s0092-8674(00)81650-5. Cell. 1999. PMID: 10535737
-
[Regulatory mechanisms of the clathrin adaptor molecules AP-1 and GGAs].Tanpakushitsu Kakusan Koso. 2008 Dec;53(16 Suppl):2046-52. Tanpakushitsu Kakusan Koso. 2008. PMID: 21038583 Review. Japanese. No abstract available.
-
The building blocks for basolateral vesicles in polarized epithelial cells.Trends Cell Biol. 2005 Apr;15(4):222-8. doi: 10.1016/j.tcb.2005.02.006. Trends Cell Biol. 2005. PMID: 15817379 Review.
Cited by
-
Recessive Mutations in AP1B1 Cause Ichthyosis, Deafness, and Photophobia.Am J Hum Genet. 2019 Nov 7;105(5):1023-1029. doi: 10.1016/j.ajhg.2019.09.021. Epub 2019 Oct 17. Am J Hum Genet. 2019. PMID: 31630788 Free PMC article.
-
Proteomic analysis of adaptor protein 1A coats selectively assembled on liposomes.Proc Natl Acad Sci U S A. 2006 Feb 28;103(9):3159-64. doi: 10.1073/pnas.0511062103. Epub 2006 Feb 21. Proc Natl Acad Sci U S A. 2006. PMID: 16492770 Free PMC article.
-
Recycling endosomes can serve as intermediates during transport from the Golgi to the plasma membrane of MDCK cells.J Cell Biol. 2004 Nov 8;167(3):531-43. doi: 10.1083/jcb.200408165. J Cell Biol. 2004. PMID: 15534004 Free PMC article.
-
The basolateral targeting signal of CD147 (EMMPRIN) consists of a single leucine and is not recognized by retinal pigment epithelium.Mol Biol Cell. 2004 Sep;15(9):4148-65. doi: 10.1091/mbc.e04-01-0058. Epub 2004 Jun 23. Mol Biol Cell. 2004. PMID: 15215314 Free PMC article.
-
Interaction of BIG2, a brefeldin A-inhibited guanine nucleotide-exchange protein, with exocyst protein Exo70.Proc Natl Acad Sci U S A. 2005 Feb 22;102(8):2784-9. doi: 10.1073/pnas.0409871102. Epub 2005 Feb 10. Proc Natl Acad Sci U S A. 2005. PMID: 15705715 Free PMC article.
References
-
- Brodsky, F.M., C.Y. Chen, C. Knuehl, M.C. Towler, and D.E. Wakeham. 2001. Biological basket weaving: formation and function of clathrin-coated vesicles. Annu. Rev. Cell Dev. Biol. 17:517–568. - PubMed
-
- Brymora, A., V.A. Valova, M.R. Larsen, B.D. Roufogalis, and P.J. Robinson. 2001. The brain exocyst complex interacts with RalA in a GTP-dependent manner: identification of a novel mammalian Sec3 gene and a second Sec15 gene. J. Biol. Chem. 276:29792–29797. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

