Interplay between TCR affinity and necessity of coreceptor ligation: high-affinity peptide-MHC/TCR interaction overcomes lack of CD8 engagement
- PMID: 14568922
- PMCID: PMC3755740
- DOI: 10.4049/jimmunol.171.9.4493
Interplay between TCR affinity and necessity of coreceptor ligation: high-affinity peptide-MHC/TCR interaction overcomes lack of CD8 engagement
Abstract
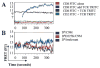
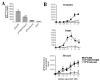
CD8 engagement is believed to be a critical event in the activation of naive T cells. In this communication, we address the effects of peptide-MHC (pMHC)/TCR affinity on the necessity of CD8 engagement in T cell activation of primary naive cells. Using two peptides with different measured avidities for the same pMHC-TCR complex, we compared biochemical affinity of pMHC/TCR and the cell surface binding avidity of pMHC/TCR with and without CD8 engagement. We compared early signaling events and later functional activity of naive T cells in the same manner. Although early signaling events are altered, we find that high-affinity pMHC/TCR interactions can overcome the need for CD8 engagement for proliferation and CTL function. An integrated signal over time allows T cell activation with a high-affinity ligand in the absence of CD8 engagement.
Figures





References
-
- Zamoyska R. CD4 and CD8: modulators of T-cell receptor recognition of antigen and of immune responses? Curr Opin Immunol. 1998;10:82. - PubMed
-
- Janeway CA., Jr The T cell receptor as a multicomponent signalling machine: CD4/CD8 coreceptors and CD45 in T cell activation. Annu Rev Immunol. 1992;10:645. - PubMed
-
- Hampl J, Chien YH, Davis MM. CD4 augments the response of a T cell to agonist but not to antagonist ligands. Immunity. 1997;7:379. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

