PPARgamma coactivator 1beta/ERR ligand 1 is an ERR protein ligand, whose expression induces a high-energy expenditure and antagonizes obesity
- PMID: 14530391
- PMCID: PMC218766
- DOI: 10.1073/pnas.2135217100
PPARgamma coactivator 1beta/ERR ligand 1 is an ERR protein ligand, whose expression induces a high-energy expenditure and antagonizes obesity
Abstract
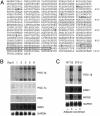
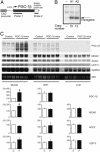
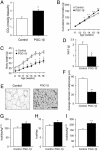
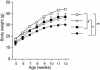
A well balanced body energy budget controlled by limitation of calorie uptake and/or increment of energy expenditure, which is typically achieved by proper physical exercise, is most effective against obesity and diabetes mellitus. Recently, peroxisome proliferator-activated receptor (PPAR) gamma, a member of the nuclear receptor, and its cofactors have been shown to be involved in lipid metabolism and in the control of energy expenditure. Here we show that PPARgamma coactivator 1 (PGC-1) beta functions as ERRL1 (for ERR ligand 1), which can bind and activate orphan ERRs (estrogen receptor-related receptors) in vitro. Consistently, PGC-1beta/ERRL1 transgenic mice exhibit increased expression of the medium-chain acyl CoA dehydrogenase, a known ERR target and a pivotal enzyme of mitochondrial beta-oxidation in skeletal muscle. As a result, the PGC-1beta/ERRL1 mice show a state similar to an athlete; namely, the mice are hyperphagic and of elevated energy expenditure and are resistant to obesity induced by a high-fat diet or by a genetic abnormality. These results demonstrate that PGC-1beta/ERRL1 can function as a protein ligand of ERR, and that its level contributes to the control of energy balance in vivo, and provide a strategy for developing novel antiobesity drugs.
Figures

Similar articles
-
Skeletal muscle and nuclear hormone receptors: implications for cardiovascular and metabolic disease.Int J Biochem Cell Biol. 2005 Oct;37(10):2047-63. doi: 10.1016/j.biocel.2005.03.002. Int J Biochem Cell Biol. 2005. PMID: 15922648 Review.
-
Role of the PGC-1 family in the metabolic adaptation of goldfish to diet and temperature.J Exp Biol. 2008 May;211(Pt 9):1448-55. doi: 10.1242/jeb.014951. J Exp Biol. 2008. PMID: 18424678
-
Loss of PGC-specific expression of the orphan nuclear receptor ERR-beta results in reduction of germ cell number in mouse embryos.Mech Dev. 2004 Mar;121(3):237-46. doi: 10.1016/j.mod.2004.01.006. Mech Dev. 2004. PMID: 15003627
-
Regulation of PPARgamma coactivator 1alpha (PGC-1alpha) signaling by an estrogen-related receptor alpha (ERRalpha) ligand.Proc Natl Acad Sci U S A. 2004 Jun 15;101(24):8912-7. doi: 10.1073/pnas.0401420101. Epub 2004 Jun 7. Proc Natl Acad Sci U S A. 2004. PMID: 15184675 Free PMC article.
-
The PGC-1/ERR signaling axis in cancer.Oncogene. 2013 Jul 25;32(30):3483-90. doi: 10.1038/onc.2012.529. Epub 2012 Dec 3. Oncogene. 2013. PMID: 23208510 Review.
Cited by
-
Complementary Roles of Estrogen-Related Receptors in Brown Adipocyte Thermogenic Function.Endocrinology. 2016 Dec;157(12):4770-4781. doi: 10.1210/en.2016-1767. Epub 2016 Oct 20. Endocrinology. 2016. PMID: 27763777 Free PMC article.
-
Peroxisome proliferator-activated receptor gamma coactivator-1 (PGC-1) family in physiological and pathophysiological process and diseases.Signal Transduct Target Ther. 2024 Mar 1;9(1):50. doi: 10.1038/s41392-024-01756-w. Signal Transduct Target Ther. 2024. PMID: 38424050 Free PMC article. Review.
-
The role of mitochondria in the pathogenesis of type 2 diabetes.Endocr Rev. 2010 Jun;31(3):364-95. doi: 10.1210/er.2009-0027. Epub 2010 Feb 15. Endocr Rev. 2010. PMID: 20156986 Free PMC article. Review.
-
PGC-1 coactivators: inducible regulators of energy metabolism in health and disease.J Clin Invest. 2006 Mar;116(3):615-22. doi: 10.1172/JCI27794. J Clin Invest. 2006. PMID: 16511594 Free PMC article. Review.
-
Erralpha and Gabpa/b specify PGC-1alpha-dependent oxidative phosphorylation gene expression that is altered in diabetic muscle.Proc Natl Acad Sci U S A. 2004 Apr 27;101(17):6570-5. doi: 10.1073/pnas.0401401101. Epub 2004 Apr 20. Proc Natl Acad Sci U S A. 2004. PMID: 15100410 Free PMC article.
References
-
- Giguere, V., Yang, N., Segui, P. & Evans, R. M. (1988) Nature 331, 91-94. - PubMed
-
- Shigeta, H., Zuo, W., Yang, N., DiAugustine, R. & Teng, C. T. (1997) J. Mol. Endocrinol. 19, 299-309. - PubMed
-
- Eudy, J. D., Yao, S., Weston, M. D., Ma-Edmonds, M., Talmadge, C. B., Cheng, J. J., Kimberling, W. J. & Sumegi, J. (1998) Genomics 50, 382-384. - PubMed
-
- Hong, H., Yang, L. & Stallcup, M. R. (1999) J. Biol. Chem. 274, 22618-22626. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

