Regulatory functions of CD8+CD28- T cells in an autoimmune disease model
- PMID: 14523041
- PMCID: PMC198520
- DOI: 10.1172/JCI17935
Regulatory functions of CD8+CD28- T cells in an autoimmune disease model
Abstract
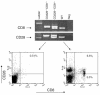
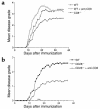
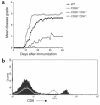
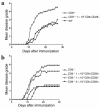
CD8+ T cell depletion renders CD28-deficient mice susceptible to experimental autoimmune encephalomyelitis (EAE). In addition, CD8-/-CD28-/- double-knockout mice are susceptible to EAE. These findings suggest a role for CD8+ T cells in the resistance of CD28-deficient mice to disease. Adoptive transfer of CD8+CD28- T cells into CD8-/- mice results in significant suppression of disease, while CD8+CD28+ T cells demonstrate no similar effect on the clinical course of EAE in the same recipients. In vitro, CD8+CD28- but not CD8+CD28+ T cells suppress IFN-gamma production of myelin oligodendrocyte glycoprotein-specific CD4+ T cells. This suppression requires cell-to-cell contact and is dependent on the presence of APCs. APCs cocultured with CD8+CD28- T cells become less efficient in inducing a T cell-dependent immune response. Such interaction prevents upregulation of costimulatory molecules by APCs, hence decreasing the delivery of these signals to CD4+ T cells. These are the first data establishing that regulatory CD8+CD28- T cells occur in normal mice and play a critical role in disease resistance in CD28-/- animals.
Figures


Similar articles
-
CD28-independent induction of experimental autoimmune encephalomyelitis.J Clin Invest. 2001 Mar;107(5):575-83. doi: 10.1172/JCI11220. J Clin Invest. 2001. PMID: 11238558 Free PMC article.
-
Studies in B7-deficient mice reveal a critical role for B7 costimulation in both induction and effector phases of experimental autoimmune encephalomyelitis.J Exp Med. 1999 Sep 6;190(5):733-40. doi: 10.1084/jem.190.5.733. J Exp Med. 1999. PMID: 10477557 Free PMC article.
-
Critical involvement of OX40 ligand signals in the T cell priming events during experimental autoimmune encephalomyelitis.J Immunol. 2001 Sep 1;167(5):2991-9. doi: 10.4049/jimmunol.167.5.2991. J Immunol. 2001. PMID: 11509650
-
Novel CD8+ Treg suppress EAE by TGF-beta- and IFN-gamma-dependent mechanisms.Eur J Immunol. 2009 Dec;39(12):3423-35. doi: 10.1002/eji.200939441. Eur J Immunol. 2009. PMID: 19768696 Free PMC article.
-
Differential requirements of naïve and memory T cells for CD28 costimulation in autoimmune pathogenesis.Histol Histopathol. 1999 Oct;14(4):1269-76. doi: 10.14670/HH-14.1269. Histol Histopathol. 1999. PMID: 10506942 Review.
Cited by
-
Donor-specific CD8+ Foxp3+ T cells protect skin allografts and facilitate induction of conventional CD4+ Foxp3+ regulatory T cells.Am J Transplant. 2012 Sep;12(9):2335-47. doi: 10.1111/j.1600-6143.2012.04120.x. Epub 2012 Jun 8. Am J Transplant. 2012. PMID: 22681667 Free PMC article.
-
Inflammatory process of CD8+ CD28- T cells in induced sputum from asthmatic patients.Mediators Inflamm. 2005 Aug 14;2005(3):160-6. doi: 10.1155/MI.2005.160. Mediators Inflamm. 2005. PMID: 16106102 Free PMC article.
-
Engagement of the CD137 (4-1BB) costimulatory molecule inhibits and reverses the autoimmune process in collagen-induced arthritis and establishes lasting disease resistance.Immunology. 2004 Sep;113(1):89-98. doi: 10.1111/j.1365-2567.2004.01952.x. Immunology. 2004. PMID: 15312139 Free PMC article.
-
Complementary contribution of CD4 and CD8 T lymphocytes to T-cell infiltration of the intact and the degenerative spinal cord.Am J Pathol. 2005 May;166(5):1441-50. doi: 10.1016/S0002-9440(10)62361-9. Am J Pathol. 2005. PMID: 15855644 Free PMC article.
-
CD40Ig treatment results in allograft acceptance mediated by CD8CD45RC T cells, IFN-gamma, and indoleamine 2,3-dioxygenase.J Clin Invest. 2007 Apr;117(4):1096-106. doi: 10.1172/JCI28801. J Clin Invest. 2007. PMID: 17404623 Free PMC article.
References
-
- Oliveira-dos-Santos AJ, et al. CD28 costimulation is crucial for the development of spontaneous autoimmune encephalomyelitis. J. Immunol. 1999;162:4490–4495. - PubMed
-
- Perrin PJ, Lavi E, Rumbley CA, Zekavat SA, Phillips SM. Experimental autoimmune meningitis: a novel neurological disease in CD28-deficient mice. Clin. Immunol. 1999;91:41–49. - PubMed
-
- Girvin AM, et al. A critical role for B7/CD28 costimulation in experimental autoimmune encephalomyelitis: a comparative study using costimulatory molecule-deficient mice and monoclonal antibody blockade. J. Immunol. 2000;164:136–143. - PubMed
-
- Karpus WJ, et al. An important role for the chemokine macrophage inflammatory protein-1 alpha in the pathogenesis of the T cell-mediated autoimmune disease, experimental autoimmune encephalomyelitis. J. Immunol. 1995;155:5003–5010. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

