LPS-TLR4 signaling to IRF-3/7 and NF-kappaB involves the toll adapters TRAM and TRIF
- PMID: 14517278
- PMCID: PMC2194210
- DOI: 10.1084/jem.20031023
LPS-TLR4 signaling to IRF-3/7 and NF-kappaB involves the toll adapters TRAM and TRIF
Erratum in
- J Exp Med. 2003 Nov 3;198(9):following 1450
Abstract
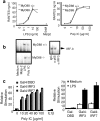
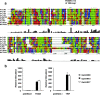
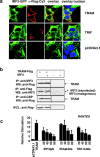
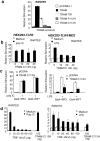
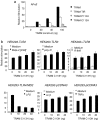
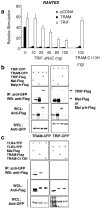
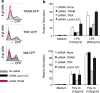
Toll-IL-1-resistance (TIR) domain-containing adaptor-inducing IFN-beta (TRIF)-related adaptor molecule (TRAM) is the fourth TIR domain-containing adaptor protein to be described that participates in Toll receptor signaling. Like TRIF, TRAM activates interferon regulatory factor (IRF)-3, IRF-7, and NF-kappaB-dependent signaling pathways. Toll-like receptor (TLR)3 and 4 activate these pathways to induce IFN-alpha/beta, regulated on activation, normal T cell expressed and secreted (RANTES), and gamma interferon-inducible protein 10 (IP-10) expression independently of the adaptor protein myeloid differentiation factor 88 (MyD88). Dominant negative and siRNA studies performed here demonstrate that TRIF functions downstream of both the TLR3 (dsRNA) and TLR4 (LPS) signaling pathways, whereas the function of TRAM is restricted to the TLR4 pathway. TRAM interacts with TRIF, MyD88 adaptor-like protein (Mal)/TIRAP, and TLR4 but not with TLR3. These studies suggest that TRIF and TRAM both function in LPS-TLR4 signaling to regulate the MyD88-independent pathway during the innate immune response to LPS.
Figures
Similar articles
-
Cutting edge: a novel Toll/IL-1 receptor domain-containing adapter that preferentially activates the IFN-beta promoter in the Toll-like receptor signaling.J Immunol. 2002 Dec 15;169(12):6668-72. doi: 10.4049/jimmunol.169.12.6668. J Immunol. 2002. PMID: 12471095
-
TIR-containing adapter molecule (TICAM)-2, a bridging adapter recruiting to toll-like receptor 4 TICAM-1 that induces interferon-beta.J Biol Chem. 2003 Dec 12;278(50):49751-62. doi: 10.1074/jbc.M305820200. Epub 2003 Sep 30. J Biol Chem. 2003. PMID: 14519765
-
Specific inhibition of MyD88-independent signaling pathways of TLR3 and TLR4 by resveratrol: molecular targets are TBK1 and RIP1 in TRIF complex.J Immunol. 2005 Sep 1;175(5):3339-46. doi: 10.4049/jimmunol.175.5.3339. J Immunol. 2005. PMID: 16116226
-
[TIR domain--containing adaptors regulate TLR-mediated signaling pathways].Nihon Rinsho. 2004 Dec;62(12):2197-203. Nihon Rinsho. 2004. PMID: 15597785 Review. Japanese.
-
TLR signaling pathways.Semin Immunol. 2004 Feb;16(1):3-9. doi: 10.1016/j.smim.2003.10.003. Semin Immunol. 2004. PMID: 14751757 Review.
Cited by
-
Phosphoinositides: Roles in the Development of Microglial-Mediated Neuroinflammation and Neurodegeneration.Front Cell Neurosci. 2021 Mar 26;15:652593. doi: 10.3389/fncel.2021.652593. eCollection 2021. Front Cell Neurosci. 2021. PMID: 33841102 Free PMC article. Review.
-
Endocytosis of Mycobacterium tuberculosis heat shock protein 60 is required to induce interleukin-10 production in macrophages.J Biol Chem. 2013 Aug 23;288(34):24956-71. doi: 10.1074/jbc.M113.461004. Epub 2013 Jul 11. J Biol Chem. 2013. PMID: 23846686 Free PMC article.
-
Adipose Tissue, Non-Communicable Diseases, and Physical Exercise: An Imperfect Triangle.Int J Mol Sci. 2023 Dec 6;24(24):17168. doi: 10.3390/ijms242417168. Int J Mol Sci. 2023. PMID: 38138997 Free PMC article. Review.
-
5,6-Dimethylxanthenone-4-acetic acid (DMXAA) activates stimulator of interferon gene (STING)-dependent innate immune pathways and is regulated by mitochondrial membrane potential.J Biol Chem. 2012 Nov 16;287(47):39776-88. doi: 10.1074/jbc.M112.382986. Epub 2012 Oct 1. J Biol Chem. 2012. PMID: 23027866 Free PMC article.
-
TRAM is involved in IL-18 signaling and functions as a sorting adaptor for MyD88.PLoS One. 2012;7(6):e38423. doi: 10.1371/journal.pone.0038423. Epub 2012 Jun 7. PLoS One. 2012. PMID: 22685567 Free PMC article.
References
-
- Medzhitov, R., P. Preston-Hurlburt, and C.A. Janeway, Jr. 1997. A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature. 388:394–397. - PubMed
-
- Akira, S. 2001. Toll-like receptors and innate immunity. Adv. Immunol. 78:1–56. - PubMed
-
- Dunne, A., and L.A. O'Neill. 2003. The interleukin-1 receptor/Toll-like receptor superfamily: signal transduction during inflammation and host defense. Sci. STKE. 2003:re3. - PubMed
-
- Muzio, M., J. Ni, P. Feng, and V.M. Dixit. 1997. IRAK (Pelle) family member IRAK-2 and MyD88 as proximal mediators of IL- 1 signaling. Science. 278:1612–1615. - PubMed
-
- Janssens, S., and R. Beyaert. 2002. A universal role for MyD88 in TLR/IL-1R-mediated signaling. Trends Biochem. Sci. 27:474–482. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

