MO25alpha/beta interact with STRADalpha/beta enhancing their ability to bind, activate and localize LKB1 in the cytoplasm
- PMID: 14517248
- PMCID: PMC204473
- DOI: 10.1093/emboj/cdg490
MO25alpha/beta interact with STRADalpha/beta enhancing their ability to bind, activate and localize LKB1 in the cytoplasm
Abstract
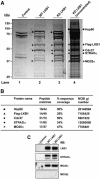
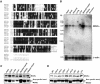
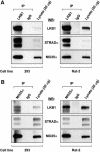
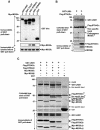
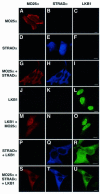
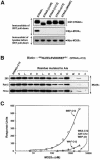
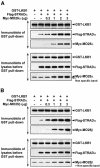
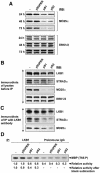
Mutations in the LKB1 protein kinase result in the inherited Peutz Jeghers cancer syndrome. LKB1 has been implicated in regulating cell proliferation and polarity although little is known about how this enzyme is regulated. We recently showed that LKB1 is activated through its interaction with STRADalpha, a catalytically deficient pseudokinase. Here we show that endogenous LKB1-STRADalpha complex is associated with a protein of unknown function, termed MO25alpha, through the interaction of MO25alpha with the last three residues of STRADalpha. MO25alpha and STRADalpha anchor LKB1 in the cytoplasm, excluding it from the nucleus. Moreover, MO25alpha enhances the formation of the LKB1-STRADalpha complex in vivo, stimulating the catalytic activity of LKB1 approximately 10-fold. We demonstrate that the related STRADbeta and MO25beta isoforms are also able to stabilize LKB1 in an active complex and that it is possible to isolate complexes of LKB1 bound to STRAD and MO25 isoforms, in which the subunits are present in equimolar amounts. Our results indicate that MO25 may function as a scaffolding component of the LKB1-STRAD complex and plays a crucial role in regulating LKB1 activity and cellular localization.
Figures


Similar articles
-
Analysis of the LKB1-STRAD-MO25 complex.J Cell Sci. 2004 Dec 15;117(Pt 26):6365-75. doi: 10.1242/jcs.01571. Epub 2004 Nov 23. J Cell Sci. 2004. PMID: 15561763
-
ATP and MO25alpha regulate the conformational state of the STRADalpha pseudokinase and activation of the LKB1 tumour suppressor.PLoS Biol. 2009 Jun 9;7(6):e1000126. doi: 10.1371/journal.pbio.1000126. Epub 2009 Jun 9. PLoS Biol. 2009. PMID: 19513107 Free PMC article.
-
Structure of the LKB1-STRAD-MO25 complex reveals an allosteric mechanism of kinase activation.Science. 2009 Dec 18;326(5960):1707-11. doi: 10.1126/science.1178377. Epub 2009 Nov 5. Science. 2009. PMID: 19892943 Free PMC article.
-
LKB1-dependent signaling pathways.Annu Rev Biochem. 2006;75:137-63. doi: 10.1146/annurev.biochem.75.103004.142702. Annu Rev Biochem. 2006. PMID: 16756488 Review.
-
LKB1 kinase: master and commander of metabolism and polarity.Curr Biol. 2004 May 25;14(10):R383-5. doi: 10.1016/j.cub.2004.05.012. Curr Biol. 2004. PMID: 15186763 Review.
Cited by
-
AMPK Causes Cell Cycle Arrest in LKB1-Deficient Cells via Activation of CAMKK2.Mol Cancer Res. 2016 Aug;14(8):683-95. doi: 10.1158/1541-7786.MCR-15-0479. Epub 2016 May 2. Mol Cancer Res. 2016. PMID: 27141100 Free PMC article.
-
Delayed diagnosis of Peutz-Jeghers syndrome due to pathological information loss or mistake in family/personal history.Orphanet J Rare Dis. 2021 Jun 8;16(1):261. doi: 10.1186/s13023-021-01900-7. Orphanet J Rare Dis. 2021. PMID: 34103092 Free PMC article.
-
Prolyl isomerase Pin1 negatively regulates AMP-activated protein kinase (AMPK) by associating with the CBS domain in the γ subunit.J Biol Chem. 2015 Oct 2;290(40):24255-66. doi: 10.1074/jbc.M115.658559. Epub 2015 Aug 14. J Biol Chem. 2015. PMID: 26276391 Free PMC article.
-
LKB1 suppresses p21-activated kinase-1 (PAK1) by phosphorylation of Thr109 in the p21-binding domain.J Biol Chem. 2010 Jun 11;285(24):18283-90. doi: 10.1074/jbc.M109.079137. Epub 2010 Apr 16. J Biol Chem. 2010. PMID: 20400510 Free PMC article.
-
miR-451 Silencing Inhibited Doxorubicin Exposure-Induced Cardiotoxicity in Mice.Biomed Res Int. 2019 Jul 4;2019:1528278. doi: 10.1155/2019/1528278. eCollection 2019. Biomed Res Int. 2019. PMID: 31355248 Free PMC article.
References
-
- Bardeesy N., Sinha,M., Hezel,A.F., Signoretti,S., Hathaway,N.A., Sharpless,N.E., Loda,M., Carrasco,D.R. and DePinho,R.A. (2002) Loss of the Lkb1 tumour suppressor provokes intestinal polyposis but resistance to transformation. Nature, 419, 162–167. - PubMed
-
- Boudeau J., Kieloch,A., Alessi,D.R., Stella,A., Guanti,G. and Resta,N. (2003b) Functional analysis of LKB1/STK11 mutants and two aberrant isoforms found in Peutz-Jeghers syndrome patients. Hum. Mutat., 21, 172. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

