Role of PACS-1 in trafficking of human cytomegalovirus glycoprotein B and virus production
- PMID: 14512558
- PMCID: PMC224974
- DOI: 10.1128/jvi.77.20.11105-11113.2003
Role of PACS-1 in trafficking of human cytomegalovirus glycoprotein B and virus production
Abstract
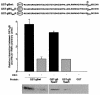
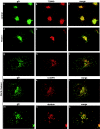
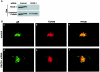
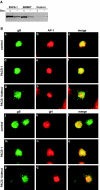
The final envelopment of herpesviruses during assembly of new virions is thought to occur by the budding of core viral particles into a late secretory pathway organelle, the trans-Golgi network (TGN), or an associated endosomal compartment. Several herpesvirus envelope glycoproteins have been previously shown to localize to the TGN when expressed independently from other viral proteins. In at least some cases this TGN localization has been shown to be dependent on clusters of acidic residues within their cytoplasmic domains. Similar acidic cluster motifs are found in endogenous membrane proteins that also localize to the TGN. These acidic cluster motifs interact with PACS-1, a connector protein that is required for the trafficking of proteins containing such motifs from endosomes to the TGN. We show here that PACS-1 interacts with the cytoplasmic domain of the HCMV envelope glycoprotein B (gB) and that PACS-1 function is required for normal TGN localization of HCMV gB. Furthermore, inhibition of PACS-1 activity in infected cells leads to a decrease in HCMV titer, whereas an increase in expression of functional PACS-1 leads to an increase in HCMV titer, suggesting that PACS-1 is required for efficient production of HCMV.
Figures
Similar articles
-
Phosphorylation of human cytomegalovirus glycoprotein B (gB) at the acidic cluster casein kinase 2 site (Ser900) is required for localization of gB to the trans-Golgi network and efficient virus replication.J Virol. 2004 Jan;78(1):285-93. doi: 10.1128/jvi.78.1.285-293.2004. J Virol. 2004. PMID: 14671110 Free PMC article.
-
Retrieval of human cytomegalovirus glycoprotein B from cell surface is not required for virus envelopment in astrocytoma cells.J Virol. 2002 May;76(10):5147-55. doi: 10.1128/jvi.76.10.5147-5155.2002. J Virol. 2002. PMID: 11967330 Free PMC article.
-
Envelopment of human cytomegalovirus occurs by budding into Golgi-derived vacuole compartments positive for gB, Rab 3, trans-golgi network 46, and mannosidase II.J Virol. 2003 Mar;77(5):3191-203. doi: 10.1128/jvi.77.5.3191-3203.2003. J Virol. 2003. PMID: 12584343 Free PMC article.
-
The role of the trans-Golgi network in varicella zoster virus biology.Cell Mol Life Sci. 2004 Dec;61(24):3047-56. doi: 10.1007/s00018-004-4269-7. Cell Mol Life Sci. 2004. PMID: 15583866 Review.
-
Human cytomegalovirus glycoprotein-receptor interactions.Transplant Proc. 1991 Jun;23(3 Suppl 3):60-3. Transplant Proc. 1991. PMID: 1648838 Review.
Cited by
-
Herpesviruses remodel host membranes for virus egress.Nat Rev Microbiol. 2011 May;9(5):382-94. doi: 10.1038/nrmicro2559. Nat Rev Microbiol. 2011. PMID: 21494278 Review.
-
Viral and host control of cytomegalovirus maturation.Trends Microbiol. 2012 Aug;20(8):392-401. doi: 10.1016/j.tim.2012.04.008. Epub 2012 May 23. Trends Microbiol. 2012. PMID: 22633075 Free PMC article. Review.
-
The phosphorylation state of an autoregulatory domain controls PACS-1-directed protein traffic.EMBO J. 2003 Dec 1;22(23):6234-44. doi: 10.1093/emboj/cdg596. EMBO J. 2003. PMID: 14633983 Free PMC article.
-
The tegument protein UL71 of human cytomegalovirus is involved in late envelopment and affects multivesicular bodies.J Virol. 2011 Apr;85(8):3821-32. doi: 10.1128/JVI.01540-10. Epub 2011 Feb 2. J Virol. 2011. PMID: 21289123 Free PMC article.
-
A Tyrosine-Based Trafficking Motif of the Tegument Protein pUL71 Is Crucial for Human Cytomegalovirus Secondary Envelopment.J Virol. 2017 Dec 14;92(1):e00907-17. doi: 10.1128/JVI.00907-17. Print 2018 Jan 1. J Virol. 2017. PMID: 29046458 Free PMC article.
References
-
- Blagoveshchenskaya, A. D., L. Thomas, S. F. Feliciangeli, C. H. Hung, and G. Thomas. 2002. HIV-1 Nef downregulates MHC-I by a PACS-1- and PI3K-regulated ARF6 endocytic pathway. Cell 111:853-866. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

