Vascular endothelial growth factor modulates skeletal myoblast function
- PMID: 14507649
- PMCID: PMC1868307
- DOI: 10.1016/S0002-9440(10)63499-2
Vascular endothelial growth factor modulates skeletal myoblast function
Abstract
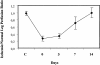
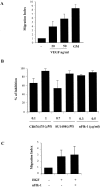
Vascular endothelial growth factor (VEGF) expression is enhanced in ischemic skeletal muscle and is thought to play a key role in the angiogenic response to ischemia. However, it is still unknown whether, in addition to new blood vessel growth, VEGF modulates skeletal muscle cell function. In the present study immunohistochemical analysis showed that, in normoperfused mouse hindlimb, VEGF and its receptors Flk-1 and Flt-1 were expressed mostly in quiescent satellite cells. Unilateral hindlimb ischemia was induced by left femoral artery ligation. At day 3 and day 7 after the induction of ischemia, Flk-1 and Flt-1 were expressed in regenerating muscle fibers and VEGF expression by these fibers was markedly enhanced. Additional in vitro experiments showed that in growing medium both cultured satellite cells and myoblast cell line C2C12 expressed VEGF and its receptors. Under these conditions, Flk-1 receptor exhibited constitutive tyrosine phosphorylation that was increased by VEGF treatment. During myogenic differentiation Flk-1 and Flt-1 were down-regulated. In a modified Boyden Chamber assay, VEGF enhanced C2C12 myoblasts migration approximately fivefold. Moreover, VEGF administration to differentiating C2C12 myoblasts prevented apoptosis, while inhibition of VEGF signaling either with selective VEGF receptor inhibitors (SU1498 and CB676475) or a neutralizing Flk-1 antibody, enhanced cell death approximately 3.5-fold. Finally, adenovirus-mediated VEGF(165) gene transfer inhibited ischemia-induced apoptosis in skeletal muscle. These results support a role for VEGF in myoblast migration and survival, and suggest a novel autocrine role of VEGF in skeletal muscle repair during ischemia.
Figures


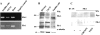
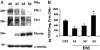

Similar articles
-
Expression of vascular endothelial growth factor and vascular endothelial growth factor receptor-2 (KDR/Flk-1) in ischemic skeletal muscle and its regeneration.Am J Pathol. 2002 Apr;160(4):1393-403. doi: 10.1016/S0002-9440(10)62566-7. Am J Pathol. 2002. PMID: 11943724 Free PMC article.
-
Differential expression of Flk-1 and Flt-1 in rat skeletal muscle in response to chronic ischaemia: favourable effect of muscle activity.Clin Sci (Lond). 2003 Oct;105(4):473-82. doi: 10.1042/CS20030035. Clin Sci (Lond). 2003. PMID: 12780346
-
Vascular endothelial growth factor expression, beta-catenin tyrosine phosphorylation, and endothelial proliferative behavior: a pathway for transformation?Lab Invest. 2003 Aug;83(8):1105-15. doi: 10.1097/01.lab.0000083531.84403.8b. Lab Invest. 2003. PMID: 12920240
-
Vascular endothelial cell growth factor (VEGF), an emerging target for cancer chemotherapy.Curr Med Chem Anticancer Agents. 2003 Mar;3(2):95-117. doi: 10.2174/1568011033353452. Curr Med Chem Anticancer Agents. 2003. PMID: 12678905 Review.
-
[Vascular endothelial growth factor--fundamental research and experimental study in plastic surgery].Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2002 Jan;16(1):64-9. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2002. PMID: 11826659 Review. Chinese.
Cited by
-
Beneficial effect of mechanical stimulation on the regenerative potential of muscle-derived stem cells is lost by inhibiting vascular endothelial growth factor.Arterioscler Thromb Vasc Biol. 2013 Aug;33(8):2004-12. doi: 10.1161/ATVBAHA.112.301166. Epub 2013 May 30. Arterioscler Thromb Vasc Biol. 2013. PMID: 23723372 Free PMC article.
-
Satellite cell-mediated angiogenesis in vitro coincides with a functional hypoxia-inducible factor pathway.Am J Physiol Cell Physiol. 2009 Jun;296(6):C1321-8. doi: 10.1152/ajpcell.00391.2008. Epub 2009 Apr 22. Am J Physiol Cell Physiol. 2009. PMID: 19386789 Free PMC article.
-
Role of protein tyrosine phosphatase 1B in vascular endothelial growth factor signaling and cell-cell adhesions in endothelial cells.Circ Res. 2008 May 23;102(10):1182-91. doi: 10.1161/CIRCRESAHA.107.167080. Epub 2008 May 1. Circ Res. 2008. PMID: 18451337 Free PMC article.
-
IGF-1 gene-modified muscle-derived stem cells are resistant to oxidative stress via enhanced activation of IGF-1R/PI3K/AKT signaling and secretion of VEGF.Mol Cell Biochem. 2014 Jan;386(1-2):167-75. doi: 10.1007/s11010-013-1855-8. Epub 2013 Oct 15. Mol Cell Biochem. 2014. PMID: 24126783
-
Strategies to promote donor cell survival: combining preconditioning approach with stem cell transplantation.J Mol Cell Cardiol. 2008 Oct;45(4):554-66. doi: 10.1016/j.yjmcc.2008.05.004. Epub 2008 May 10. J Mol Cell Cardiol. 2008. PMID: 18561945 Free PMC article. Review.
References
-
- Matsumoto T, Claesson-Welsh L: VEGF receptor signal transduction. Sci STKE 2001, 112:1-17 - PubMed
-
- Kalka C, Masuda H, Takahashi T, Gordon R, Tepper O, Gravereaux E, Pieczek A, Iwaguro H, Hayashi SI, Isner JM, Asahara T: Vascular endothelial growth factor(165) gene transfer augments circulating endothelial progenitor cells in human subjects. Circ Res 2000, 86:1198-1202 - PubMed
-
- Kalka C, Tehrani H, Laudenberg B, Vale PR, Isner JM, Asahara T, Symes JF: VEGF gene transfer mobilizes endothelial progenitor cells in patients with inoperable coronary disease. Ann Thorac Surg 2000, 70:829-834 - PubMed
-
- Tischer E, Mitchell R, Hartman T, Silva M, Gospodarowicz D, Fiddes JC, Abraham JA: The human gene for vascular endothelial growth factor: multiple protein forms are encoded through alternative exon splicing. J Biol Chem 1991, 266:11947-11954 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

