The proinflammatory mediators C3a and C5a are essential for liver regeneration
- PMID: 12975457
- PMCID: PMC2194207
- DOI: 10.1084/jem.20030374
The proinflammatory mediators C3a and C5a are essential for liver regeneration
Abstract
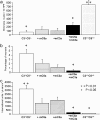
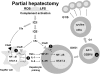
Complement has been implicated in liver repair after toxic injury. Here, we demonstrate that complement components are essential for liver regeneration, and mediate their effect by interacting with key signaling networks that promote hepatocyte proliferation. C3- or C5-deficient mice exhibited high mortality, parenchymal damage, and impaired liver regeneration after partial hepatectomy. Mice with dual C3 and C5 deficiency had a more exacerbated phenotype that was reversed by combined C3a and C5a reconstitution. Interception of C5a receptor signaling resulted in suppression of IL-6/TNFalpha induction and lack of C3 and C5a receptor stimulation attenuated nuclear factor-kappaB/STAT-3 activation after hepatectomy. These data indicate that C3a and C5a, two potent inflammatory mediators of the innate immune response, contribute essentially to the early priming stages of hepatocyte regeneration.
Figures


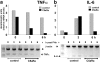

Similar articles
-
A novel role of complement: mice deficient in the fifth component of complement (C5) exhibit impaired liver regeneration.J Immunol. 2001 Feb 15;166(4):2479-86. doi: 10.4049/jimmunol.166.4.2479. J Immunol. 2001. PMID: 11160308
-
Immune cell-derived C3a and C5a costimulate human T cell alloimmunity.Am J Transplant. 2013 Oct;13(10):2530-9. doi: 10.1111/ajt.12405. Epub 2013 Sep 6. Am J Transplant. 2013. PMID: 24033923 Free PMC article.
-
Targeting C3a/C5a receptors inhibits human mesangial cell proliferation and alleviates immunoglobulin A nephropathy in mice.Clin Exp Immunol. 2017 Jul;189(1):60-70. doi: 10.1111/cei.12961. Epub 2017 Apr 10. Clin Exp Immunol. 2017. PMID: 28295247 Free PMC article.
-
The Complement C3a and C5a Signaling in Renal Diseases: A Bridge between Acute and Chronic Inflammation.Nephron. 2024;148(10):712-723. doi: 10.1159/000538241. Epub 2024 Mar 8. Nephron. 2024. PMID: 38452744 Review.
-
Complement in central nervous system inflammation.Immunol Res. 2002;26(1-3):7-13. doi: 10.1385/IR:26:1-3:007. Immunol Res. 2002. PMID: 12403340 Review.
Cited by
-
C5a receptor signaling prevents folate deficiency-induced neural tube defects in mice.J Immunol. 2013 Apr 1;190(7):3493-9. doi: 10.4049/jimmunol.1203072. Epub 2013 Feb 18. J Immunol. 2013. PMID: 23420882 Free PMC article.
-
Complement activation and inhibition in wound healing.Clin Dev Immunol. 2012;2012:534291. doi: 10.1155/2012/534291. Epub 2012 Dec 30. Clin Dev Immunol. 2012. PMID: 23346185 Free PMC article. Review.
-
Rapid, Dynamic Activation of Müller Glial Stem Cell Responses in Zebrafish.Invest Ophthalmol Vis Sci. 2016 Oct 1;57(13):5148-5160. doi: 10.1167/iovs.16-19973. Invest Ophthalmol Vis Sci. 2016. PMID: 27699411 Free PMC article.
-
Transcriptional control of complement receptor gene expression.Immunol Res. 2007;39(1-3):146-59. doi: 10.1007/s12026-007-0078-z. Immunol Res. 2007. PMID: 17917062 Review.
-
Complement and neutrophil function changes after liver resection in humans.World J Surg. 2009 Dec;33(12):2635-43. doi: 10.1007/s00268-009-0209-x. World J Surg. 2009. PMID: 19789912
References
-
- Mastellos, D., and J.D. Lambris. 2002. Complement: more than a ‘guard’ against invading pathogens? Trends Immunol. 23:485–491. - PubMed
-
- Reca, R., D. Mastellos, M. Majka, L. Marquez, J. Ratajczak, S. Franchini, A. Glodek, M. Honczarenko, L.A. Spruce, A. Janowska-Wieczorek, et al. 2003. Functional receptor for C3a anaphylatoxin is expressed by normal hematopoietic stem/progenitor cells, and C3a enhances their homing-related responses to SDF-1. Blood. 101:3784–3793. - PubMed
-
- Rio-Tsonis, K., P.A. Tsonis, I.K. Zarkadis, A.G. Tsagas, and J.D. Lambris. 1998. Expression of the third component of complement, C3, in regenerating limb blastema cells of urodeles. J. Immunol. 161:6819–6824. - PubMed
-
- Kimura, Y., M. Madhavan, M.K. Call, W. Santiago, P.A. Tsonis, J.D. Lambris, and K. Rio-Tsonis. 2003. Expression of complement 3 and complement 5 in newt limb and lens regeneration. J. Immunol. 170:2331–2339. - PubMed
-
- Michalopoulos, G.K., and M.C. DeFrances. 1997. Liver regeneration. Science. 276:60–66. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

