Rer1p, a retrieval receptor for ER membrane proteins, recognizes transmembrane domains in multiple modes
- PMID: 12972550
- PMCID: PMC196553
- DOI: 10.1091/mbc.e02-12-0777
Rer1p, a retrieval receptor for ER membrane proteins, recognizes transmembrane domains in multiple modes
Abstract
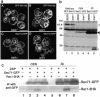
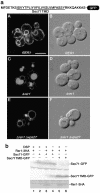
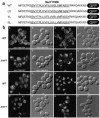
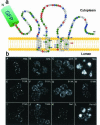
The yeast Golgi membrane protein Rer1p is required for the retrieval of various endoplasmic reticulum (ER) membrane proteins such as Sec12p and Sec71p to the ER. We demonstrate here that the transmembrane domain (TMD) of Sec71p, a type-III membrane protein, contains an ER localization signal, which is required for physical recognition by Rer1p. The Sec71TMD-GFP fusion protein is efficiently retrieved to the ER by Rer1p. The structural feature of this TMD signal turns out to be the spatial location of polar residues flanking the highly hydrophobic core sequence but not the whole length of the TMD. On the Rer1p side, Tyr152 residue in the 4th TMD is important for the recognition of Sec12p but not Sec71p, suggesting that Rer1p interacts with its ligands at least in two modes. Sec71TMD-GFP expressed in the Deltarer1 mutant cells is mislocalized from the ER to the lumen of vacuoles via the multivesicular body (MVB) sorting pathway. In this case, not only the presence of polar residues in the Sec71TMD but also the length of the TMD is critical for the MVB sorting. Thus, the Rer1p-dependent ER retrieval and the MVB sorting in late endosomes both watch polar residues in the TMD but in a different manner.
Figures




Similar articles
-
Endoplasmic reticulum localization of Sec12p is achieved by two mechanisms: Rer1p-dependent retrieval that requires the transmembrane domain and Rer1p-independent retention that involves the cytoplasmic domain.J Cell Biol. 1996 Jul;134(2):279-93. doi: 10.1083/jcb.134.2.279. J Cell Biol. 1996. PMID: 8707815 Free PMC article.
-
Rer1p, a retrieval receptor for endoplasmic reticulum membrane proteins, is dynamically localized to the Golgi apparatus by coatomer.J Cell Biol. 2001 Mar 5;152(5):935-44. doi: 10.1083/jcb.152.5.935. J Cell Biol. 2001. PMID: 11238450 Free PMC article.
-
Membrane protein retrieval from the Golgi apparatus to the endoplasmic reticulum (ER): characterization of the RER1 gene product as a component involved in ER localization of Sec12p.Mol Biol Cell. 1995 Nov;6(11):1459-77. doi: 10.1091/mbc.6.11.1459. Mol Biol Cell. 1995. PMID: 8589449 Free PMC article.
-
[The mechanisms of endoplasmic reticulum protein localization by vesicle recycling].Seikagaku. 1998 Dec;70(12):1387-400. Seikagaku. 1998. PMID: 10025160 Review. Japanese. No abstract available.
-
Transmembrane Domain Recognition during Membrane Protein Biogenesis and Quality Control.Curr Biol. 2018 Apr 23;28(8):R498-R511. doi: 10.1016/j.cub.2018.02.004. Curr Biol. 2018. PMID: 29689233 Review.
Cited by
-
Endoplasmic Reticulum Export of GPI-Anchored Proteins.Int J Mol Sci. 2019 Jul 17;20(14):3506. doi: 10.3390/ijms20143506. Int J Mol Sci. 2019. PMID: 31319476 Free PMC article. Review.
-
The Golgi Localization of GnTI Requires a Polar Amino Acid Residue within Its Transmembrane Domain.Plant Physiol. 2019 Jun;180(2):859-873. doi: 10.1104/pp.19.00310. Epub 2019 Apr 10. Plant Physiol. 2019. PMID: 30971450 Free PMC article.
-
Static retention of the lumenal monotopic membrane protein torsinA in the endoplasmic reticulum.EMBO J. 2011 Jul 22;30(16):3217-31. doi: 10.1038/emboj.2011.233. EMBO J. 2011. PMID: 21785409 Free PMC article.
-
Misfolded proteins are sorted by a sequential checkpoint mechanism of ER quality control.J Cell Biol. 2004 Apr;165(1):41-52. doi: 10.1083/jcb.200309132. J Cell Biol. 2004. PMID: 15078901 Free PMC article.
-
Protein quality control in the secretory pathway.J Cell Biol. 2019 Oct 7;218(10):3171-3187. doi: 10.1083/jcb.201906047. Epub 2019 Sep 19. J Cell Biol. 2019. PMID: 31537714 Free PMC article. Review.
References
-
- Bonifacino, J.S., Cosson, P., and Klausner, R.D. (1990a). Colocalized transmembrane determinants for ER degradation and subunit assembly explain the intracellular fate of TCR chains. Cell 63, 503–513. - PubMed
-
- Bonifacino, J.S., Suzuki, C.K., and Klausner, R.D. (1990b). A peptide sequence confers retention and rapid degradation in the endoplasmic reticulum. Science 247, 79–82. - PubMed
-
- Camirand, A., Heysen, A., Grondin, B., and Herscovics, A. (1991). Glycoprotein biosynthesis in Saccharomyces cerevisiae. Isolation and characterization of the gene encoding a specific processing α-mannosidase. J. Biol. Chem. 266, 15120–15127. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

