Hypermethylation of the human glutathione S-transferase-pi gene (GSTP1) CpG island is present in a subset of proliferative inflammatory atrophy lesions but not in normal or hyperplastic epithelium of the prostate: a detailed study using laser-capture microdissection
- PMID: 12937133
- PMCID: PMC1868250
- DOI: 10.1016/s0002-9440(10)63452-9
Hypermethylation of the human glutathione S-transferase-pi gene (GSTP1) CpG island is present in a subset of proliferative inflammatory atrophy lesions but not in normal or hyperplastic epithelium of the prostate: a detailed study using laser-capture microdissection
Abstract
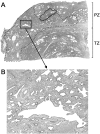
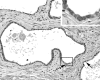
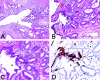
Somatic inactivation of the glutathione S-transferase-pi gene (GSTP1) via CpG island hypermethylation occurs early during prostate carcinogenesis, present in approximately 70% of high-grade prostatic intraepithelial neoplasia (high-grade PIN) lesions and more than 90% of adenocarcinomas. Recently, there has been a resurgence of the concept that foci of prostatic atrophy (referred to as proliferative inflammatory atrophy or PIA) may be precursor lesions for the development of prostate cancer and/or high-grade PIN. Many of the cells within PIA lesions contain elevated levels of GSTP1, glutathione S-transferase-alpha (GSTA1), and cyclooxygenase-II proteins, suggesting a stress response. Because not all PIA cells are positive for GSTP1 protein, we hypothesized that some of the cells within these regions acquire GSTP1 CpG island hypermethylation, increasing the chance of progression to high-grade PIN and/or adenocarcinoma. Separate regions (n =199) from 27 formalin-fixed paraffin-embedded prostates were microdissected by laser-capture microdissection (Arcturus PixCell II). These regions included normal epithelium (n = 48), hyperplasticepithelium from benign prostatic hyperplasia nodules (n = 22), PIA (n = 64), high-grade PIN (n = 32), and adenocarcinoma (n = 33). Genomic DNA was isolated and assessed for GSTP1 CpG island hypermethylation by methylation-specific polymerase chain reaction. GSTP1 CpG island hypermethylation was not detected in normal epithelium (0 of 48) or in hyperplastic epithelium (0 of 22), but was found in 4 of 64 (6.3%) PIA lesions. The difference in the frequency of GSTP1 CpG island hypermethylation between normal or hyperplastic epithelium and PIA was statistically significant (P = 0.049). Similar to studies using nonmicrodissected cases, hypermethylation was found in 22 of 32 (68.8%) high-grade PIN lesions and in 30 of 33 (90.9%) adenocarcinoma lesions. Unlike normal or hyperplastic epithelium, GSTP1 CpG island hypermethylation can be detected in some PIA lesions. These data support the hypothesis that atrophic epithelium in a subset of PIA lesions may lead to high-grade PIN and/or adenocarcinoma. Because these atrophic lesions are so prevalent and extensive, even though only a small subset contains this somatic DNA alteration, the clinical impact may be substantial.
Figures



Similar articles
-
Progressive Spreading of DNA Methylation in the GSTP1 Promoter CpG Island across Transitions from Precursors to Invasive Prostate Cancer.Cancer Prev Res (Phila). 2023 Aug 1;16(8):449-460. doi: 10.1158/1940-6207.CAPR-22-0485. Cancer Prev Res (Phila). 2023. PMID: 37347938 Free PMC article.
-
GSTP1 CpG island hypermethylation as a molecular biomarker for prostate cancer.J Cell Biochem. 2004 Feb 15;91(3):540-52. doi: 10.1002/jcb.10740. J Cell Biochem. 2004. PMID: 14755684 Review.
-
Preneoplastic prostate lesions: an opportunity for prostate cancer prevention.Ann N Y Acad Sci. 2001 Dec;952:135-44. doi: 10.1111/j.1749-6632.2001.tb02734.x. Ann N Y Acad Sci. 2001. PMID: 11795433 Review.
-
Reversal of GSTP1 CpG island hypermethylation and reactivation of pi-class glutathione S-transferase (GSTP1) expression in human prostate cancer cells by treatment with procainamide.Cancer Res. 2001 Dec 15;61(24):8611-6. Cancer Res. 2001. PMID: 11751372
-
Proliferative inflammatory atrophy of the prostate: implications for prostatic carcinogenesis.Am J Pathol. 1999 Dec;155(6):1985-92. doi: 10.1016/S0002-9440(10)65517-4. Am J Pathol. 1999. PMID: 10595928 Free PMC article.
Cited by
-
Genomic and epigenomic alterations in prostate cancer.Front Endocrinol (Lausanne). 2012 Nov 6;3:128. doi: 10.3389/fendo.2012.00128. eCollection 2012. Front Endocrinol (Lausanne). 2012. PMID: 23133437 Free PMC article.
-
Potential Role of Epigenetic Mechanism in Manganese Induced Neurotoxicity.Biomed Res Int. 2016;2016:2548792. doi: 10.1155/2016/2548792. Epub 2016 May 26. Biomed Res Int. 2016. PMID: 27314012 Free PMC article. Review.
-
Prostate cancer and inflammation: the evidence.Histopathology. 2012 Jan;60(1):199-215. doi: 10.1111/j.1365-2559.2011.04033.x. Histopathology. 2012. PMID: 22212087 Free PMC article. Review.
-
Analysis of DNA methylation of multiple genes in microdissected cells from formalin-fixed and paraffin-embedded tissues.J Histochem Cytochem. 2009 May;57(5):477-89. doi: 10.1369/jhc.2009.953026. Epub 2009 Jan 19. J Histochem Cytochem. 2009. PMID: 19153192 Free PMC article.
-
DNA Methylation-Mediated Downregulation of DEFB1 in Prostate Cancer Cells.PLoS One. 2016 Nov 11;11(11):e0166664. doi: 10.1371/journal.pone.0166664. eCollection 2016. PLoS One. 2016. PMID: 27835705 Free PMC article.
References
-
- Putzi MJ, De Marzo AM: Prostate pathology: histologic and molecular perspectives. Hematol Oncol Clin North Am 2001, 15:407-421 - PubMed
-
- Ruska KM, Sauvageot J, Epstein JI: Histology and cellular kinetics of prostatic atrophy. Am J Surg Pathol 1998, 22:1073-1077 - PubMed
-
- McNeal JE: Normal histology of the prostate. Am J Surg Pathol 1988, 12:619-633 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

