Sodium channel beta4, a new disulfide-linked auxiliary subunit with similarity to beta2
- PMID: 12930796
- PMCID: PMC6740763
- DOI: 10.1523/JNEUROSCI.23-20-07577.2003
Sodium channel beta4, a new disulfide-linked auxiliary subunit with similarity to beta2
Abstract
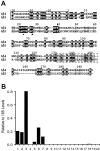
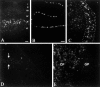
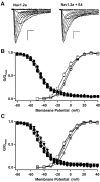
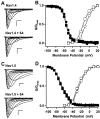
The principal alpha subunit of voltage-gated sodium channels is associated with auxiliary beta subunits that modify channel function and mediate protein-protein interactions. We have identified a new beta subunit termed beta4. Like the beta1-beta3 subunits, beta4 contains a cleaved signal sequence, an extracellular Ig-like fold, a transmembrane segment, and a short intracellular C-terminal tail. Using TaqMan reverse transcription-PCR analysis, in situ hybridization, and immunocytochemistry, we show that beta4 is widely distributed in neurons in the brain, spinal cord, and some sensory neurons.beta4 is most similar to the beta2 subunit (35% identity), and, like the beta2 subunit, the Ig-like fold of beta4 contains an unpaired cysteine that may interact with the alpha subunit. Under nonreducing conditions, beta4 has a molecular mass exceeding 250 kDa because of its covalent linkage to Nav1.2a, whereas on reduction, it migrates with a molecular mass of 38 kDa, similar to the mature glycosylated forms of the other beta subunits. Coexpression of beta4 with brain Nav1.2a and skeletal muscle Nav1.4 alpha subunits in tsA-201 cells resulted in a negative shift in the voltage dependence of channel activation, which overrode the opposite effects of beta1 and beta3 subunits when they were present. This novel, disulfide-linked beta subunit is likely to affect both protein-protein interactions and physiological function of multiple sodium channel alpha subunits.
Figures





Similar articles
-
Regulation of persistent Na current by interactions between beta subunits of voltage-gated Na channels.J Neurosci. 2009 Feb 18;29(7):2027-42. doi: 10.1523/JNEUROSCI.4531-08.2009. J Neurosci. 2009. PMID: 19228957 Free PMC article.
-
Isoform-specific effects of the beta2 subunit on voltage-gated sodium channel gating.J Biol Chem. 2006 Sep 8;281(36):25875-81. doi: 10.1074/jbc.M605060200. Epub 2006 Jul 17. J Biol Chem. 2006. PMID: 16847056
-
Molecular cloning and functional expression of the human sodium channel beta1B subunit, a novel splicing variant of the beta1 subunit.Eur J Biochem. 2003 Dec;270(23):4762-70. doi: 10.1046/j.1432-1033.2003.03878.x. Eur J Biochem. 2003. PMID: 14622265
-
Sodium channel auxiliary subunits.J Mol Microbiol Biotechnol. 2007;12(3-4):249-62. doi: 10.1159/000099646. J Mol Microbiol Biotechnol. 2007. PMID: 17587873 Review.
-
Molecular properties of brain sodium channels: an important target for anticonvulsant drugs.Adv Neurol. 1999;79:441-56. Adv Neurol. 1999. PMID: 10514834 Review.
Cited by
-
Α- and β-subunit composition of voltage-gated sodium channels investigated with μ-conotoxins and the recently discovered μO§-conotoxin GVIIJ.J Neurophysiol. 2015 Apr 1;113(7):2289-301. doi: 10.1152/jn.01004.2014. Epub 2015 Jan 28. J Neurophysiol. 2015. PMID: 25632083 Free PMC article.
-
Voltage-gated sodium channels at 60: structure, function and pathophysiology.J Physiol. 2012 Jun 1;590(11):2577-89. doi: 10.1113/jphysiol.2011.224204. Epub 2012 Apr 2. J Physiol. 2012. PMID: 22473783 Free PMC article. Review.
-
Neurological perspectives on voltage-gated sodium channels.Brain. 2012 Sep;135(Pt 9):2585-612. doi: 10.1093/brain/aws225. Brain. 2012. PMID: 22961543 Free PMC article. Review.
-
On the multiple roles of the voltage gated sodium channel β1 subunit in genetic diseases.Front Pharmacol. 2015 May 18;6:108. doi: 10.3389/fphar.2015.00108. eCollection 2015. Front Pharmacol. 2015. PMID: 26042039 Free PMC article. Review.
-
NaV1.6 and NaV1.7 channels are major endogenous voltage-gated sodium channels in ND7/23 cells.PLoS One. 2019 Aug 16;14(8):e0221156. doi: 10.1371/journal.pone.0221156. eCollection 2019. PLoS One. 2019. PMID: 31419255 Free PMC article.
References
-
- Catterall WA ( 2000) From ionic currents to molecular mechanisms: the structure and function of voltage-gated sodium channels. Neuron 26: 13-25. - PubMed
-
- Escayg A, MacDonald BT, Meisler MH, Baulac S, Huberfeld G, An-Gourfinkel I, Brice A, LeGuern E, Moulard B, Chaigne D, Buresi C, Malafosse A ( 2000) Mutations of SCN1A, encoding a neuronal sodium channel, in two families with GEFS+2. Nat Genet 24: 343-345. - PubMed
-
- Eubanks J, Srinivasan J, Dinulos MB, Disteche CM, Catterall WA ( 1997) Structure and chromosomal localization of the β2 subunit of the human brain sodium channel. NeuroReport 8: 2775-2779. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
