Trafficking of prion proteins through a caveolae-mediated endosomal pathway
- PMID: 12925711
- PMCID: PMC2173792
- DOI: 10.1083/jcb.200304140
Trafficking of prion proteins through a caveolae-mediated endosomal pathway
Abstract
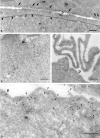
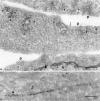
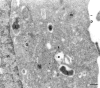
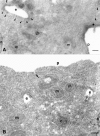
To understand the posttranslational conversion of the cellular prion protein (PrPC) to its pathologic conformation, it is important to define the intracellular trafficking pathway of PrPC within the endomembrane system. We studied the localization and internalization of PrPC in CHO cells using cryoimmunogold electron microscopy. At steady state, PrPC was enriched in caveolae both at the TGN and plasma membrane and in interconnecting chains of endocytic caveolae. Protein A-gold particles bound specifically to PrPC on live cells. These complexes were delivered via caveolae to the pericentriolar region and via nonclassical, caveolae-containing early endocytic structures to late endosomes/lysosomes, thereby bypassing the internalization pathway mediated by clathrin-coated vesicles. Endocytosed PrPC-containing caveolae were not directed to the ER and Golgi complex. Uptake of caveolae and degradation of PrPC was slow and sensitive to filipin. This caveolae-dependent endocytic pathway was not observed for several other glycosylphosphatidyl inositol (GPI)-anchored proteins. We propose that this nonclassical endocytic pathway is likely to determine the subcellular location of PrPC conversion.
Figures






Similar articles
-
A glycolipid-anchored prion protein is endocytosed via clathrin-coated pits.J Cell Biol. 1994 Jun;125(6):1239-50. doi: 10.1083/jcb.125.6.1239. J Cell Biol. 1994. PMID: 7911471 Free PMC article.
-
Ligand-induced caveolae-mediated internalization of A1 adenosine receptors: morphological evidence of endosomal sorting and receptor recycling.Exp Cell Res. 2003 Apr 15;285(1):72-90. doi: 10.1016/s0014-4827(02)00090-3. Exp Cell Res. 2003. PMID: 12681288
-
Dual control of caveolar membrane traffic by microtubules and the actin cytoskeleton.J Cell Sci. 2002 Nov 15;115(Pt 22):4327-39. doi: 10.1242/jcs.00117. J Cell Sci. 2002. PMID: 12376564
-
PrPc on the road: trafficking of the cellular prion protein.J Neurochem. 2004 Feb;88(4):769-81. doi: 10.1046/j.1471-4159.2003.02199.x. J Neurochem. 2004. PMID: 14756798 Review.
-
Caveosomes and endocytosis of lipid rafts.J Cell Sci. 2003 Dec 1;116(Pt 23):4707-14. doi: 10.1242/jcs.00840. J Cell Sci. 2003. PMID: 14600257 Review.
Cited by
-
PRNP/prion protein regulates the secretion of exosomes modulating CAV1/caveolin-1-suppressed autophagy.Autophagy. 2016 Nov;12(11):2113-2128. doi: 10.1080/15548627.2016.1226735. Epub 2016 Sep 14. Autophagy. 2016. PMID: 27629560 Free PMC article.
-
Prions.Cold Spring Harb Perspect Biol. 2011 Jan 1;3(1):a006833. doi: 10.1101/cshperspect.a006833. Cold Spring Harb Perspect Biol. 2011. PMID: 21421910 Free PMC article. Review.
-
Cholesterol balance in prion diseases and Alzheimer's disease.Viruses. 2014 Nov 20;6(11):4505-35. doi: 10.3390/v6114505. Viruses. 2014. PMID: 25419621 Free PMC article. Review.
-
An Update on Autophagy in Prion Diseases.Front Bioeng Biotechnol. 2020 Aug 27;8:975. doi: 10.3389/fbioe.2020.00975. eCollection 2020. Front Bioeng Biotechnol. 2020. PMID: 32984276 Free PMC article. Review.
-
HIV-1 Tat enters T cells using coated pits before translocating from acidified endosomes and eliciting biological responses.Mol Biol Cell. 2004 May;15(5):2347-60. doi: 10.1091/mbc.e03-12-0921. Epub 2004 Mar 12. Mol Biol Cell. 2004. PMID: 15020715 Free PMC article.
References
-
- Anderson, R.G. 1998. The caveolae membrane system. Annu. Rev. Biochem. 67:199–225. - PubMed
-
- Arnold, J.E., C. Tipler, L. Laszlo, J. Hope, M. Landon, and R.J. Mayer. 1995. The abnormal isoform of the prion protein accumulates in late-endosome-like organelles in scrapie-infected mouse brain. J. Pathol. 176:403–411. - PubMed
-
- Blochberger, T.C., C. Cooper, D. Peretz, J. Tatzelt, O.H. Griffith, M.A. Baldwin, and S.B. Prusiner. 1997. Prion protein expression in Chinese hamster ovary cells using a glutamine synthetase selection and amplification system. Protein Eng. 10:1465–1473. - PubMed
-
- Bouillot, C., A. Prochiantz, G. Rougon, and B. Allinquant. 1996. Axonal amyloid precursor protein expressed by neurons in vitro is present in a membrane fraction with caveolae-like properties. J. Biol. Chem. 271:7640–7644. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

