Inhibition of nucleo-cytoplasmic trafficking by RNA viruses: targeting the nuclear pore complex
- PMID: 12921994
- PMCID: PMC7125697
- DOI: 10.1016/s0168-1702(03)00165-5
Inhibition of nucleo-cytoplasmic trafficking by RNA viruses: targeting the nuclear pore complex
Abstract
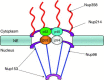
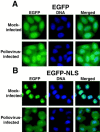
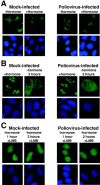
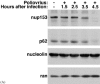
Analysis of virus-host interactions has revealed a variety of ways in which viruses utilize and/or alter host functions in an effort to facilitate efficient replication. Recent work has suggested that certain RNA viruses that replicate in the cytoplasm disrupt the normal trafficking of cellular RNAs and proteins within the host cell. This review will examine the recent evidence showing that poliovirus and vesicular stomatitis virus (VSV) can inhibit nucleo-cytoplasmic transport within cells. Interestingly, the data indicate that inhibition by both viruses involves targeting components of the nuclear pore complex (NPC). Following this, several possible explanations for why viruses might disrupt nucleo-cytoplasmic transport are discussed. Finally, the possibility that disruption of nucleo-cytoplasmic trafficking may be a more common feature of RNA virus-host interactions than previously thought is examined.
Figures

Similar articles
-
Effects of poliovirus infection on nucleo-cytoplasmic trafficking and nuclear pore complex composition.EMBO J. 2001 Jan 15;20(1-2):240-9. doi: 10.1093/emboj/20.1.240. EMBO J. 2001. PMID: 11226174 Free PMC article.
-
Viral subversion of nucleocytoplasmic trafficking.Traffic. 2014 Feb;15(2):127-40. doi: 10.1111/tra.12137. Epub 2013 Dec 2. Traffic. 2014. PMID: 24289861 Free PMC article. Review.
-
Inhibition of nuclear import and alteration of nuclear pore complex composition by rhinovirus.J Virol. 2002 Sep;76(17):8787-96. doi: 10.1128/jvi.76.17.8787-8796.2002. J Virol. 2002. PMID: 12163599 Free PMC article.
-
The Nuclear Pore Complex Is a Key Target of Viral Proteases to Promote Viral Replication.Viruses. 2021 Apr 19;13(4):706. doi: 10.3390/v13040706. Viruses. 2021. PMID: 33921849 Free PMC article. Review.
-
Nuclear pore blockade reveals that HIV-1 completes reverse transcription and uncoating in the nucleus.Nat Microbiol. 2020 Sep;5(9):1088-1095. doi: 10.1038/s41564-020-0735-8. Epub 2020 Jun 1. Nat Microbiol. 2020. PMID: 32483230 Free PMC article.
Cited by
-
Viral subversion of host functions for picornavirus translation and RNA replication.Future Virol. 2012 Feb;7(2):179-191. doi: 10.2217/fvl.12.2. Future Virol. 2012. PMID: 23293659 Free PMC article.
-
Rhinovirus protease cleavage of nucleoporins: perspective on implications for airway remodeling.Front Microbiol. 2024 Jan 5;14:1321531. doi: 10.3389/fmicb.2023.1321531. eCollection 2023. Front Microbiol. 2024. PMID: 38249483 Free PMC article.
-
HIV-1 remodels the nuclear pore complex.J Cell Biol. 2011 May 16;193(4):619-31. doi: 10.1083/jcb.201008064. J Cell Biol. 2011. PMID: 21576391 Free PMC article.
-
Enterovirus D68 molecular and cellular biology and pathogenesis.J Biol Chem. 2021 Jan-Jun;296:100317. doi: 10.1016/j.jbc.2021.100317. Epub 2021 Jan 21. J Biol Chem. 2021. PMID: 33484714 Free PMC article. Review.
-
Double-stranded RNA is produced by positive-strand RNA viruses and DNA viruses but not in detectable amounts by negative-strand RNA viruses.J Virol. 2006 May;80(10):5059-64. doi: 10.1128/JVI.80.10.5059-5064.2006. J Virol. 2006. PMID: 16641297 Free PMC article.
References
-
- Adam S.A., Sterne-Marr R., Gerace L. Nuclear protein import using digitonin-permeabilized cells. Methods Enzymol. 1992;219:97–110. - PubMed
-
- Allen T.D., Cronshaw J.M., Bagley S., Kiseleva E., Goldberg M.W. The nuclear pore complex: mediator of translocation between nucleus and cytoplasm. J. Cell. Sci. 2000;113(Pt 10):1651–1659. - PubMed
-
- Alonso-Caplen F.V., Nemeroff M.E., Qiu Y., Krug R.M. Nucleocytoplasmic transport: the influenza virus NS1 protein regulates the transport of spliced NS2 mRNA and its precursor NS1 mRNA. Genes Dev. 1992;6:255–267. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

