Cell cycle regulation by Kaposi's sarcoma-associated herpesvirus K-bZIP: direct interaction with cyclin-CDK2 and induction of G1 growth arrest
- PMID: 12915577
- PMCID: PMC187423
- DOI: 10.1128/jvi.77.17.9652-9661.2003
Cell cycle regulation by Kaposi's sarcoma-associated herpesvirus K-bZIP: direct interaction with cyclin-CDK2 and induction of G1 growth arrest
Abstract
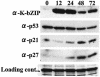
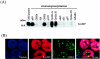
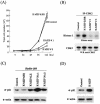
In order to cope with hostile host environments, many viruses have developed strategies to perturb the cellular machinery to suit their replication needs. Some herpesvirus genes protect cells from undergoing apoptosis to prolong the lives of infected cells, while others, such as Epstein-Barr virus Zta, slow down the G(1)/S transition phase to allow ample opportunity for transcription and translation of viral genes before the onset of cellular genomic replication. In this study, we investigated whether Kaposi's sarcoma-associated herpesvirus (KSHV) K-bZIP, a homologue of the Epstein-Barr virus transcription factor BZLF1 (Zta), plays a role in cell cycle regulation. Here we show that K-bZIP physically associates with cyclin-CDK2 and downmodulates its kinase activity. The association can be detected in the natural environment of KSHV-infected cells without artificial overexpression of either component. With purified protein, it can be shown that the interaction between K-bZIP and cyclin-CDK2 is direct and that K-bZIP alone is sufficient to inhibit CDK2 activity. The interacting domain of K-bZIP has been mapped to the basic region. The result of these associations is a prolonged G(1) phase, accompanied by the induction of p21 and p27 in a naturally infected B-cell line. Thus, in addition to the previously described transcription and genome replication functions, a new role of K-bZIP in KSHV replication is identified in this report.
Figures


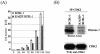

Similar articles
-
Kaposi's sarcoma-associated herpesvirus K-bZIP is a coregulator of K-Rta: physical association and promoter-dependent transcriptional repression.J Virol. 2003 Jan;77(2):1441-51. doi: 10.1128/jvi.77.2.1441-1451.2003. J Virol. 2003. PMID: 12502859 Free PMC article.
-
CCAAT/enhancer-binding protein-alpha is induced during the early stages of Kaposi's sarcoma-associated herpesvirus (KSHV) lytic cycle reactivation and together with the KSHV replication and transcription activator (RTA) cooperatively stimulates the viral RTA, MTA, and PAN promoters.J Virol. 2003 Sep;77(17):9590-612. doi: 10.1128/jvi.77.17.9590-9612.2003. J Virol. 2003. PMID: 12915572 Free PMC article.
-
Modulation of p27(Kip1) levels by the cyclin encoded by Kaposi's sarcoma-associated herpesvirus.EMBO J. 1999 Feb 1;18(3):654-63. doi: 10.1093/emboj/18.3.654. EMBO J. 1999. PMID: 9927425 Free PMC article.
-
bZIP proteins of human gammaherpesviruses.J Gen Virol. 2003 Aug;84(Pt 8):1941-1949. doi: 10.1099/vir.0.19112-0. J Gen Virol. 2003. PMID: 12867624 Review.
-
The bZIP Proteins of Oncogenic Viruses.Viruses. 2020 Jul 14;12(7):757. doi: 10.3390/v12070757. Viruses. 2020. PMID: 32674309 Free PMC article. Review.
Cited by
-
Sleeping Beauty transposase modulates cell-cycle progression through interaction with Miz-1.Proc Natl Acad Sci U S A. 2006 Mar 14;103(11):4062-7. doi: 10.1073/pnas.0507683103. Epub 2006 Mar 7. Proc Natl Acad Sci U S A. 2006. PMID: 16537485 Free PMC article.
-
SARS-CoV-2 hijacks cellular kinase CDK2 to promote viral RNA synthesis.Signal Transduct Target Ther. 2022 Dec 27;7(1):400. doi: 10.1038/s41392-022-01239-w. Signal Transduct Target Ther. 2022. PMID: 36575184 Free PMC article.
-
Productive herpesvirus lytic replication in primary effusion lymphoma cells requires S-phase entry.J Gen Virol. 2020 Aug;101(8):873-883. doi: 10.1099/jgv.0.001444. J Gen Virol. 2020. PMID: 32501196 Free PMC article.
-
Breaking Bad: How Viruses Subvert the Cell Cycle.Front Cell Infect Microbiol. 2018 Nov 19;8:396. doi: 10.3389/fcimb.2018.00396. eCollection 2018. Front Cell Infect Microbiol. 2018. PMID: 30510918 Free PMC article. Review.
-
Kaposi's Sarcoma-Associated Herpesvirus K8 Is an RNA Binding Protein That Regulates Viral DNA Replication in Coordination with a Noncoding RNA.J Virol. 2018 Mar 14;92(7):e02177-17. doi: 10.1128/JVI.02177-17. Print 2018 Apr 1. J Virol. 2018. PMID: 29321307 Free PMC article.
References
-
- Alkan, S., D. S. Karcher, A. Ortiz, S. Khalil, M. Akhtar, and M. A. Ali. 1997. Human herpesvirus-8/Kaposi's sarcoma-associated herpesvirus in organ transplant patients with immunosuppression. Br. J. Haematol. 96:412-414. - PubMed
-
- Bartek, J., and J. Lukas. 2001. Pathways governing G1/S transition and their response to DNA damage. FEBS Lett. 490:117-122. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

