HIF prolyl-hydroxylase 2 is the key oxygen sensor setting low steady-state levels of HIF-1alpha in normoxia
- PMID: 12912907
- PMCID: PMC175782
- DOI: 10.1093/emboj/cdg392
HIF prolyl-hydroxylase 2 is the key oxygen sensor setting low steady-state levels of HIF-1alpha in normoxia
Abstract
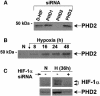
Hypoxia-inducible factor (HIF), a transcriptional complex conserved from Caenorhabditis elegans to vertebrates, plays a pivotal role in cellular adaptation to low oxygen availability. In normoxia, the HIF-alpha subunits are targeted for destruction by prolyl hydroxylation, a specific modification that provides recognition for the E3 ubiquitin ligase complex containing the von Hippel-Lindau tumour suppressor protein (pVHL). Three HIF prolyl-hydroxylases (PHD1, 2 and 3) were identified recently in mammals and shown to hydroxylate HIF-alpha subunits. Here we show that specific 'silencing' of PHD2 with short interfering RNAs is sufficient to stabilize and activate HIF-1alpha in normoxia in all the human cells investigated. 'Silencing' of PHD1 and PHD3 has no effect on the stability of HIF-1alpha either in normoxia or upon re-oxygenation of cells briefly exposed to hypoxia. We therefore conclude that, in vivo, PHDs have distinct assigned functions, PHD2 being the critical oxygen sensor setting the low steady-state levels of HIF-1alpha in normoxia. Interestingly, PHD2 is upregulated by hypoxia, providing an HIF-1-dependent auto-regulatory mechanism driven by the oxygen tension.
Figures






Similar articles
-
Hypoxia-inducible factor-1 (HIF-1) promotes its degradation by induction of HIF-alpha-prolyl-4-hydroxylases.Biochem J. 2004 Aug 1;381(Pt 3):761-7. doi: 10.1042/BJ20040620. Biochem J. 2004. PMID: 15104534 Free PMC article.
-
Regulation of HIF prolyl hydroxylases by hypoxia-inducible factors.J Cell Biochem. 2004 Jun 1;92(3):491-501. doi: 10.1002/jcb.20067. J Cell Biochem. 2004. PMID: 15156561
-
Differential function of the prolyl hydroxylases PHD1, PHD2, and PHD3 in the regulation of hypoxia-inducible factor.J Biol Chem. 2004 Sep 10;279(37):38458-65. doi: 10.1074/jbc.M406026200. Epub 2004 Jul 7. J Biol Chem. 2004. PMID: 15247232
-
Regulation of HIF by the von Hippel-Lindau tumour suppressor: implications for cellular oxygen sensing.IUBMB Life. 2001 Jul;52(1-2):43-7. doi: 10.1080/15216540252774757. IUBMB Life. 2001. PMID: 11795592 Review.
-
HIF hydroxylation and cellular oxygen sensing.Biol Chem. 2004 Mar-Apr;385(3-4):223-30. doi: 10.1515/BC.2004.016. Biol Chem. 2004. PMID: 15134335 Review.
Cited by
-
Exposure to cigarette smoke induces overexpression of von Hippel-Lindau tumor suppressor in mouse skeletal muscle.Am J Physiol Lung Cell Mol Physiol. 2012 Sep 15;303(6):L519-27. doi: 10.1152/ajplung.00007.2012. Epub 2012 Jul 27. Am J Physiol Lung Cell Mol Physiol. 2012. PMID: 22842216 Free PMC article.
-
The Role of 2-Oxoglutarate Dependent Dioxygenases in Gliomas and Glioblastomas: A Review of Epigenetic Reprogramming and Hypoxic Response.Front Oncol. 2021 Mar 25;11:619300. doi: 10.3389/fonc.2021.619300. eCollection 2021. Front Oncol. 2021. PMID: 33842321 Free PMC article. Review.
-
Changes in Hypoxia-Inducible Factor-1 (HIF-1) and Regulatory Prolyl Hydroxylase (PHD) Enzymes Following Hypoxic-Ischemic Injury in the Neonatal Rat.Neurochem Res. 2016 Mar;41(3):515-22. doi: 10.1007/s11064-015-1641-y. Epub 2015 Jun 25. Neurochem Res. 2016. PMID: 26108712
-
The E3 ubiquitin-protein ligase MDM2 is a novel interactor of the von Hippel-Lindau tumor suppressor.Sci Rep. 2020 Sep 28;10(1):15850. doi: 10.1038/s41598-020-72683-3. Sci Rep. 2020. PMID: 32985545 Free PMC article.
-
ATF4 and HIF-1α in bone: an intriguing relationship.J Bone Miner Res. 2013 Sep;28(9):1866-9. doi: 10.1002/jbmr.2045. J Bone Miner Res. 2013. PMID: 23873659 Free PMC article. No abstract available.
References
-
- Berra E., Richard,D.E., Gothié,E. and Pouysségur,J. (2001a) HIF-1-dependent transcriptional activity is required for oxygen-mediated HIF-1α degradation. FEBS Lett., 491, 85–90. - PubMed
-
- Brondello J.M., Brunet,A., Pouysségur,J. and McKenzie,F.R. (1997) The dual specificity mitogen-activated protein kinase phosphatase-1 and -2 are induced by the p42/p44MAPK cascade. J. Biol. Chem., 272, 1368–1376. - PubMed
-
- Bruick R.K. and McKnight,S.L. (2001) A conserved family of prolyl-4-hydroxylases that modify HIF. Science, 294, 1337–1340. - PubMed
-
- Chan D.A., Sutphin,P.D., Denko,N.C. and Giaccia,A.J. (2002) Role of prolyl hydroxylation in oncogenically stabilized hypoxia-inducible factor-1α. J. Biol. Chem., 277, 40112–40117. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

