Paranodal interactions regulate expression of sodium channel subtypes and provide a diffusion barrier for the node of Ranvier
- PMID: 12904461
- PMCID: PMC6740666
- DOI: 10.1523/JNEUROSCI.23-18-07001.2003
Paranodal interactions regulate expression of sodium channel subtypes and provide a diffusion barrier for the node of Ranvier
Abstract
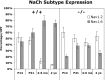
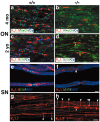
The node of Ranvier is a distinct domain of myelinated axons that is highly enriched in sodium channels and is critical for impulse propagation. During development, the channel subtypes expressed at the node undergo a transition from Nav1.2 to Nav1.6. Specialized junctions that form between the paranodal glial membranes and axon flank the nodes and are candidates to regulate their maturation and delineate their boundaries. To investigate these roles, we characterized node development in mice deficient in contactin-associated protein (Caspr), an integral junctional component. Paranodes in these mice lack transverse bands, a hallmark of the mature junction, and exhibit progressive disruption of axon-paranodal loop interactions in the CNS. Caspr mutant mice display significant abnormalities at central nodes; components of the nodes progressively disperse along axons, and many nodes fail to mature properly, persistently expressing Nav1.2 rather than Nav1.6. In contrast, PNS nodes are only modestly longer and, although maturation is delayed, eventually all express Nav1.6. Potassium channels are aberrantly clustered in the paranodes; these clusters are lost over time in the CNS, whereas they persist in the PNS. These findings indicate that interactions of the paranodal loops with the axon promote the transition in sodium channel subtypes at CNS nodes and provide a lateral diffusion barrier that, even in the absence of transverse bands, maintains a high concentration of components at the node and the integrity of voltage-gated channel domains.
Figures


Similar articles
-
Localization of Caspr2 in myelinated nerves depends on axon-glia interactions and the generation of barriers along the axon.J Neurosci. 2001 Oct 1;21(19):7568-75. doi: 10.1523/JNEUROSCI.21-19-07568.2001. J Neurosci. 2001. PMID: 11567047 Free PMC article.
-
Paranodal axoglial junction is required for the maintenance of the Nav1.6-type sodium channel in the node of Ranvier in the optic nerves but not in peripheral nerve fibers in the sulfatide-deficient mice.Glia. 2004 May;46(3):274-83. doi: 10.1002/glia.20008. Glia. 2004. PMID: 15048850
-
Contactin orchestrates assembly of the septate-like junctions at the paranode in myelinated peripheral nerve.Neuron. 2001 May;30(2):385-97. doi: 10.1016/s0896-6273(01)00296-3. Neuron. 2001. PMID: 11395001
-
Nodes of Ranvier come of age.Trends Neurosci. 2002 Jan;25(1):2-5. doi: 10.1016/s0166-2236(00)02006-3. Trends Neurosci. 2002. PMID: 11801321 Review.
-
Molecular organization and function of vertebrate septate-like junctions.Biochim Biophys Acta Biomembr. 2020 May 1;1862(5):183211. doi: 10.1016/j.bbamem.2020.183211. Epub 2020 Feb 4. Biochim Biophys Acta Biomembr. 2020. PMID: 32032590 Review.
Cited by
-
Glial ensheathment of peripheral axons in Drosophila.J Neurosci Res. 2008 May 1;86(6):1189-98. doi: 10.1002/jnr.21574. J Neurosci Res. 2008. PMID: 18041093 Free PMC article. Review.
-
Development of a model for microphysiological simulations: small nodes of ranvier from peripheral nerves of mice reconstructed by electron tomography.Neuroinformatics. 2005;3(2):133-62. doi: 10.1385/NI:3:2:133. Neuroinformatics. 2005. PMID: 15988042
-
Altered ion channels in an animal model of Charcot-Marie-Tooth disease type IA.J Neurosci. 2005 Feb 9;25(6):1470-80. doi: 10.1523/JNEUROSCI.3328-04.2005. J Neurosci. 2005. PMID: 15703401 Free PMC article.
-
Pathophysiology of the Different Clinical Phenotypes of Chronic Inflammatory Demyelinating Polyradiculoneuropathy (CIDP).Int J Mol Sci. 2021 Dec 24;23(1):179. doi: 10.3390/ijms23010179. Int J Mol Sci. 2021. PMID: 35008604 Free PMC article. Review.
-
The axon-glia unit in white matter stroke: mechanisms of damage and recovery.Brain Res. 2015 Oct 14;1623:123-34. doi: 10.1016/j.brainres.2015.02.019. Epub 2015 Feb 20. Brain Res. 2015. PMID: 25704204 Free PMC article. Review.
References
-
- Arroyo EJ, Xu YT, Zhou L, Messing A, Peles E, Chiu SY, Scherer SS ( 1999) Myelinating Schwann cells determine the internodal localization of Kv1.1, Kv1.2, Kvbeta2, and Caspr. J Neurocytol 28: 333-347. - PubMed
-
- Bennett V, Lambert S ( 1999) Physiological roles of axonal ankyrins in survival of premyelinated axons and localization of voltage-gated sodium channels. J Neurocytol 28: 303-318. - PubMed
-
- Berghs S, Aggujaro D, Dirkx Jr R, Maksimova E, Stabach P, Hermel JM, Zhang JP, Philbrick W, Slepnev V, Ort T, Solimena M ( 2000) BetaIV spectrin, a new spectrin localized at axon initial segments and nodes of Ranvier in the central and peripheral nervous system. J Cell Biol 151: 985-1002. - PMC - PubMed
-
- Bhat MA, Rios JC, Lu Y, Garcia-Fresco GP, Ching W, Martin MS, Li J, Einheber S, Chesler M, Rosenbluth J, Salzer JL, Bellen HJ ( 2001) Axon-glia interactions and the domain organization of myelinated axons requires Neurexin IV/Caspr/Paranodin. Neuron 30: 369-383. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
