HSP27 is a ubiquitin-binding protein involved in I-kappaBalpha proteasomal degradation
- PMID: 12897149
- PMCID: PMC166315
- DOI: 10.1128/MCB.23.16.5790-5802.2003
HSP27 is a ubiquitin-binding protein involved in I-kappaBalpha proteasomal degradation
Abstract
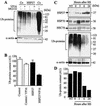
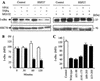
HSP27 is an ATP-independent chaperone that confers protection against apoptosis through various mechanisms, including a direct interaction with cytochrome c. Here we show that HSP27 overexpression in various cell types enhances the degradation of ubiquitinated proteins by the 26S proteasome in response to stressful stimuli, such as etoposide or tumor necrosis factor alpha (TNF-alpha). We demonstrate that HSP27 binds to polyubiquitin chains and to the 26S proteasome in vitro and in vivo. The ubiquitin-proteasome pathway is involved in the activation of transcription factor NF-kappaB by degrading its main inhibitor, I-kappaBalpha. HSP27 overexpression increases NF-kappaB nuclear relocalization, DNA binding, and transcriptional activity induced by etoposide, TNF-alpha, and interleukin 1beta. HSP27 does not affect I-kappaBalpha phosphorylation but enhances the degradation of phosphorylated I-kappaBalpha by the proteasome. The interaction of HSP27 with the 26S proteasome is required to activate the proteasome and the degradation of phosphorylated I-kappaBalpha. A protein complex that includes HSP27, phosphorylated I-kappaBalpha, and the 26S proteasome is formed. Based on these observations, we propose that HSP27, under stress conditions, favors the degradation of ubiquitinated proteins, such as phosphorylated I-kappaBalpha. This novel function of HSP27 would account for its antiapoptotic properties through the enhancement of NF-kappaB activity.
Figures
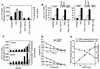

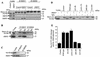


Similar articles
-
A novel in vitro assay for deubiquitination of I kappa B alpha.Arch Biochem Biophys. 2002 Apr 1;400(1):76-84. doi: 10.1006/abbi.2002.2760. Arch Biochem Biophys. 2002. PMID: 11913973
-
Ubiquitin/proteasome-dependent degradation of D-type cyclins is linked to tumor necrosis factor-induced cell cycle arrest.J Biol Chem. 2002 May 10;277(19):16528-37. doi: 10.1074/jbc.M109929200. Epub 2002 Feb 25. J Biol Chem. 2002. PMID: 11864973
-
Transient nuclear factor kappaB (NF-kappaB) activation stimulated by interleukin-1beta may be partly dependent on proteasome activity, but not phosphorylation and ubiquitination of the IkappaBalpha molecule, in C6 glioma cells. Regulation of NF-kappaB linked to chemokine production.J Biol Chem. 1999 May 28;274(22):15875-82. doi: 10.1074/jbc.274.22.15875. J Biol Chem. 1999. PMID: 10336492
-
Proteolytic signaling by TNFalpha: caspase activation and IkappaB degradation.Cytokine. 2003 Mar 21;21(6):286-94. doi: 10.1016/s1043-4666(03)00107-8. Cytokine. 2003. PMID: 12824002 Review.
-
Control of IkappaBalpha proteolysis by the ubiquitin-proteasome pathway.Biochimie. 2001 Mar-Apr;83(3-4):351-6. doi: 10.1016/s0300-9084(01)01237-8. Biochimie. 2001. PMID: 11295496 Review.
Cited by
-
HSC70 regulates cold-induced caspase-1 hyperactivation by an autoinflammation-causing mutant of cytoplasmic immune receptor NLRC4.Proc Natl Acad Sci U S A. 2019 Oct 22;116(43):21694-21703. doi: 10.1073/pnas.1905261116. Epub 2019 Oct 9. Proc Natl Acad Sci U S A. 2019. PMID: 31597739 Free PMC article.
-
Molecular insights of exercise therapy in disease prevention and treatment.Signal Transduct Target Ther. 2024 May 29;9(1):138. doi: 10.1038/s41392-024-01841-0. Signal Transduct Target Ther. 2024. PMID: 38806473 Free PMC article. Review.
-
Prognostic impact of c-Rel nuclear expression and REL amplification and crosstalk between c-Rel and the p53 pathway in diffuse large B-cell lymphoma.Oncotarget. 2015 Sep 15;6(27):23157-80. doi: 10.18632/oncotarget.4319. Oncotarget. 2015. PMID: 26324762 Free PMC article.
-
NF-κB signaling is essential for resistance to heat stress-induced early stage apoptosis in human umbilical vein endothelial cells.Sci Rep. 2015 Sep 4;5:13547. doi: 10.1038/srep13547. Sci Rep. 2015. PMID: 26337463 Free PMC article.
-
Repeated Rounds of Gonadotropin Stimulation Induce Imbalance in the Antioxidant Machinery and Activation of Pro-Survival Proteins in Mouse Oviducts.Int J Mol Sci. 2023 May 26;24(11):9294. doi: 10.3390/ijms24119294. Int J Mol Sci. 2023. PMID: 37298244 Free PMC article.
References
-
- Adams, J. 2002. Proteasome inhibitors as new anticancer drugs. Curr. Opin. Oncol. 14:628-634. - PubMed
-
- Baldwin, A. S., Jr. 1996. The NF-kappa B and I kappa B proteins: new discoveries and insights. Annu. Rev. Immunol. 14:649-683. - PubMed
-
- Benaroudj, N., and A. L. Goldberg. 2000. PAN, the proteasome-activating nucleotidase from archaebacteria, is a protein-unfolding molecular chaperone. Nat. Cell Biol. 2:833-839. - PubMed
-
- Bender, A. T., D. R. Demady, and Y. Osawa. 2000. Ubiquitination of neuronal nitric-oxide synthase in vitro and in vivo. J. Biol. Chem. 275:17407-17411. - PubMed
-
- Boelens, W. C., Y. Croes, and W. W. de Jong. 2001. Interaction between alphaB-crystallin and the human 20S proteasomal subunit C8/alpha7. Biochim. Biophys. Acta 1544:311-319. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
