Potential role for ADAM15 in pathological neovascularization in mice
- PMID: 12897135
- PMCID: PMC166329
- DOI: 10.1128/MCB.23.16.5614-5624.2003
Potential role for ADAM15 in pathological neovascularization in mice
Abstract
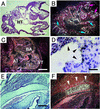
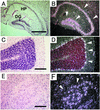
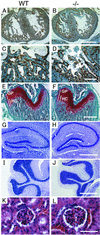
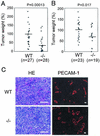
ADAM15 (named for a disintegrin and metalloprotease 15, metargidin) is a membrane-anchored glycoprotein that has been implicated in cell-cell or cell-matrix interactions and in the proteolysis of molecules on the cell surface or extracellular matrix. To characterize the potential roles of ADAM15 during development and in adult mice, we analyzed its expression pattern by mRNA in situ hybridization and generated mice carrying a targeted deletion of ADAM15 (adam15(-/-) mice). A high level of expression of ADAM15 was found in vascular cells, the endocardium, hypertrophic cells in developing bone, and specific areas of the hippocampus and cerebellum. However, despite the pronounced expression of ADAM15 in these tissues, no major developmental defects or pathological phenotypes were evident in adam15(-/-) mice. The elevated levels of ADAM15 in endothelial cells prompted an evaluation of its role in neovascularization. In a mouse model for retinopathy of prematurity, adam15(-/-) mice had a major reduction in neovascularization compared to wild-type controls. Furthermore, the size of tumors resulting from implanted B16F0 mouse melanoma cells was significantly smaller in adam15(-/-) mice than in wild-type controls. Since ADAM15 does not appear to be required for developmental angiogenesis or for adult homeostasis, it may represent a novel target for the design of inhibitors of pathological neovascularization.
Figures



Similar articles
-
Characterization of oxygen-induced retinopathy in mice carrying an inactivating point mutation in the catalytic site of ADAM15.Invest Ophthalmol Vis Sci. 2014 Sep 23;55(10):6774-82. doi: 10.1167/iovs.14-14472. Invest Ophthalmol Vis Sci. 2014. PMID: 25249606 Free PMC article.
-
Homeostatic effects of the metalloproteinase disintegrin ADAM15 in degenerative cartilage remodeling.Arthritis Rheum. 2005 Apr;52(4):1100-9. doi: 10.1002/art.20974. Arthritis Rheum. 2005. PMID: 15818704
-
An Adam15 amplification loop promotes vascular endothelial growth factor-induced ocular neovascularization.FASEB J. 2008 Aug;22(8):2775-83. doi: 10.1096/fj.07-099283. Epub 2008 Apr 1. FASEB J. 2008. PMID: 18381816 Free PMC article.
-
The role of the disintegrin metalloproteinase ADAM15 in prostate cancer progression.J Cell Biochem. 2009 Apr 15;106(6):967-74. doi: 10.1002/jcb.22087. J Cell Biochem. 2009. PMID: 19229865 Review.
-
Role of the vascular endothelial growth factor isoforms in retinal angiogenesis and DiGeorge syndrome.Verh K Acad Geneeskd Belg. 2005;67(4):229-76. Verh K Acad Geneeskd Belg. 2005. PMID: 16334858 Review.
Cited by
-
Expression of ADAM-15 in rat myocardial infarction.Int J Exp Pathol. 2009 Jun;90(3):347-54. doi: 10.1111/j.1365-2613.2009.00642.x. Int J Exp Pathol. 2009. PMID: 19563617 Free PMC article.
-
The ADAM metalloproteinases.Mol Aspects Med. 2008 Oct;29(5):258-89. doi: 10.1016/j.mam.2008.08.001. Epub 2008 Aug 15. Mol Aspects Med. 2008. PMID: 18762209 Free PMC article. Review.
-
Identification of membrane proteins regulated by ADAM15 by SUSPECS proteomics.Front Mol Biosci. 2023 Jun 14;10:1162504. doi: 10.3389/fmolb.2023.1162504. eCollection 2023. Front Mol Biosci. 2023. PMID: 37388246 Free PMC article.
-
A disintegrin and metalloproteinase 15-mediated glycocalyx shedding contributes to vascular leakage during inflammation.Cardiovasc Res. 2018 Nov 1;114(13):1752-1763. doi: 10.1093/cvr/cvy167. Cardiovasc Res. 2018. PMID: 29939250 Free PMC article.
-
Proteomic-based detection of a protein cluster dysregulated during cardiovascular development identifies biomarkers of congenital heart defects.PLoS One. 2009;4(1):e4221. doi: 10.1371/journal.pone.0004221. Epub 2009 Jan 19. PLoS One. 2009. PMID: 19156209 Free PMC article.
References
-
- Abe, M., and Y. Sato. 2001. cDNA microarray analysis of the gene expression profile of VEGF-activated human umbilical vein endothelial cells. Angiogenesis 4:289-298. - PubMed
-
- Alfandari, D., H. Cousin, A. Gaultier, K. Smith, J. M. White, T. Darribere, and D. W. DeSimone. 2001. Xenopus ADAM 13 is a metalloprotease required for cranial neural crest-cell migration. Curr. Biol. 11:918-930. - PubMed
-
- Asakura, M., M. Kitakaze, S. Takashima, Y. Liao, F. Ishikura, T. Yoshinaka, H. Ohmoto, K. Node, K. Yoshino, H. Ishiguro, H. Asanuma, S. Sanada, Y. Matsumura, H. Takeda, S. Beppu, M. Tada, M. Hori, and S. Higashiyama. 2002. Cardiac hypertrophy is inhibited by antagonism of ADAM12 processing of HB-EGF: metalloproteinase inhibitors as a new therapy. Nat. Med. 8:35-40. - PubMed
-
- Black, R. A., C. T. Rauch, C. J. Kozlosky, J. J. Peschon, J. L. Slack, M. F. Wolfson, B. J. Castner, K. L. Stocking, P. Reddy, S. Srinivasan, N. Nelson, N. Boiani, K. A. Schooley, M. Gerhart, R. Davis, J. N. Fitzner, R. S. Johnson, R. J. Paxton, C. J. March, and D. P. Cerretti. 1997. A metalloproteinase disintegrin that releases tumour-necrosis factor-α from cells. Nature 385:729-733. - PubMed
-
- Black, R. A., and J. M. White. 1998. ADAMs: focus on the protease domain. Curr. Opin. Cell Biol. 10:654-659. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
