Beta-amyloid regulation of presynaptic nicotinic receptors in rat hippocampus and neocortex
- PMID: 12890766
- PMCID: PMC6740736
- DOI: 10.1523/JNEUROSCI.23-17-06740.2003
Beta-amyloid regulation of presynaptic nicotinic receptors in rat hippocampus and neocortex
Abstract
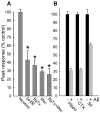
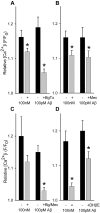
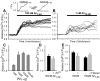
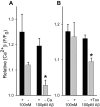
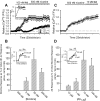
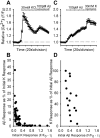
Alteration by beta-amyloid (Abeta) of signaling via nicotinic acetylcholine receptors (nAChRs) has been implicated in the early stages of Alzheimer's disease. nAChRs function both post- and presynaptically in the nervous system; however, little is known about the functional consequence of the interaction of Abeta with these receptors, particularly those on presynaptic nerve terminals. In view of the strong correlation between loss of synaptic terminals and dementia, together with the reduction in nAChRs in Alzheimer's disease, the possibility exists that presynaptic nAChRs may be targets for Abeta. To explore this possibility, we assessed the effect of Abeta peptides on nicotine-evoked changes in presynaptic Ca2+ level via confocal imaging of isolated presynaptic nerve endings from rat hippocampus and neocortex. Abeta1-42 appeared to inhibit presynaptic nAChR activation by nicotine. Surprisingly, picomolar Abeta1-42 was found to directly evoke sustained increases in presynaptic Ca2+ via nAChRs, revealing that the apparent inhibitory action of Abeta1-42 was the result of an occlusion of nicotine to further stimulate the receptors. The direct effect of Abeta was found to be sensitive to alpha-bungarotoxin, mecamylamine, and dihydro-beta-erythroidine, indicating involvement of alpha7-containing nAChRs and non-alpha7-containing nAChRs. Prior depolarization strongly attenuated subsequent Abeta-evoked responses in a manner dependent on the amplitude of the initial presynaptic Ca2+ increase, suggesting that nerve activity or Ca2+ channel density may control the impact of Abeta on presynaptic nerve terminal function. Together, these results suggest that the sustained increases in presynaptic Ca2+ evoked by Abeta may underlie disruptions in neuronal signaling via nAChRs in the early stages of Alzheimer's disease.
Figures

Similar articles
-
Direct evidence that release-stimulating alpha7* nicotinic cholinergic receptors are localized on human and rat brain glutamatergic axon terminals.J Neurochem. 2002 Mar;80(6):1071-8. doi: 10.1046/j.0022-3042.2002.00805.x. J Neurochem. 2002. PMID: 11953457
-
P2X(7) receptors exert a permissive role on the activation of release-enhancing presynaptic alpha7 nicotinic receptors co-existing on rat neocortex glutamatergic terminals.Neuropharmacology. 2006 May;50(6):705-13. doi: 10.1016/j.neuropharm.2005.11.016. Epub 2006 Jan 19. Neuropharmacology. 2006. PMID: 16427662
-
Beta-amyloid activates the mitogen-activated protein kinase cascade via hippocampal alpha7 nicotinic acetylcholine receptors: In vitro and in vivo mechanisms related to Alzheimer's disease.J Neurosci. 2001 Jun 15;21(12):4125-33. doi: 10.1523/JNEUROSCI.21-12-04125.2001. J Neurosci. 2001. PMID: 11404397 Free PMC article.
-
On the interaction of β-amyloid peptides and α7-nicotinic acetylcholine receptors in Alzheimer's disease.Curr Alzheimer Res. 2013 Jul;10(6):618-30. doi: 10.2174/15672050113109990132. Curr Alzheimer Res. 2013. PMID: 23627750 Review.
-
Modulatory role of presynaptic nicotinic receptors in synaptic and non-synaptic chemical communication in the central nervous system.Brain Res Brain Res Rev. 1999 Nov;30(3):219-35. doi: 10.1016/s0165-0173(99)00016-8. Brain Res Brain Res Rev. 1999. PMID: 10567725 Review.
Cited by
-
The toxicity of amyloid β oligomers.Int J Mol Sci. 2012;13(6):7303-7327. doi: 10.3390/ijms13067303. Epub 2012 Jun 13. Int J Mol Sci. 2012. PMID: 22837695 Free PMC article. Review.
-
Mossy fiber long-term potentiation deficits in BACE1 knock-outs can be rescued by activation of alpha7 nicotinic acetylcholine receptors.J Neurosci. 2010 Oct 13;30(41):13808-13. doi: 10.1523/JNEUROSCI.1070-10.2010. J Neurosci. 2010. PMID: 20943921 Free PMC article.
-
Distinct in vivo roles of secreted APP ectodomain variants APPsα and APPsβ in regulation of spine density, synaptic plasticity, and cognition.EMBO J. 2018 Jun 1;37(11):e98335. doi: 10.15252/embj.201798335. Epub 2018 Apr 16. EMBO J. 2018. PMID: 29661886 Free PMC article.
-
Allosteric modulator Desformylflustrabromine relieves the inhibition of α2β2 and α4β2 nicotinic acetylcholine receptors by β-amyloid(1-42) peptide.J Mol Neurosci. 2011 Sep;45(1):42-7. doi: 10.1007/s12031-011-9509-3. Epub 2011 Mar 22. J Mol Neurosci. 2011. PMID: 21424792 Free PMC article.
-
Nicotinic ACh receptors as therapeutic targets in CNS disorders.Trends Pharmacol Sci. 2015 Feb;36(2):96-108. doi: 10.1016/j.tips.2014.12.002. Epub 2015 Jan 29. Trends Pharmacol Sci. 2015. PMID: 25639674 Free PMC article. Review.
References
-
- Arias HR ( 1997) Topology of ligand binding sites on the nicotinic acetylcholine receptor. Brain Res Rev 25: 133-191. - PubMed
-
- Auld DS, Kar S, Quirion R ( 1998) β-Amyloid peptides as direct cholinergic neuromodulators: a missing link? Trends Neurosci 21: 43-49. - PubMed
-
- Brendza RP, O'Brien C, Simmons K, McKeel DW, Bales KR, Paul SM, Olney JW, Sanes JR, Holtzman DM ( 2003) PDAPP; YFP double transgenic mice: a tool to study amyloid-associated changes in axonal, dendritic, and synaptic structures. J Comp Neurol 456: 375-383. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous
