The early progenitors of mouse dendritic cells and plasmacytoid predendritic cells are within the bone marrow hemopoietic precursors expressing Flt3
- PMID: 12874262
- PMCID: PMC2194069
- DOI: 10.1084/jem.20030107
The early progenitors of mouse dendritic cells and plasmacytoid predendritic cells are within the bone marrow hemopoietic precursors expressing Flt3
Abstract
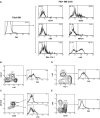
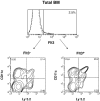
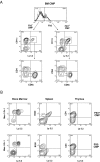
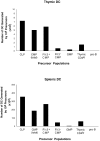
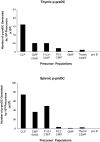
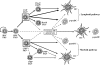
Flt3 ligand (Flt3L) is a growth factor for hemopoietic progenitors and can promote the expansion of both conventional dendritic cells (DCs) and plasmacytoid predendritic cells (p-preDCs). The cells responding to Flt3L treatment and the precursors for the DCs and p-preDCs had not been fully characterized. We examined different mouse bone marrow (BM) hemopoietic precursor populations for the surface expression of Flt3 and tested them for early DC and p-preDC precursor activity. Most DC precursor activity, other than that given by multipotent hemopoietic stem cells, was within the downstream precursors expressing Flt3. The majority of mouse BM common lymphoid precursors expressed high levels of Flt3 and these were the most efficient precursors of both DCs and p-preDCs. In contrast, only a small proportion of the common myeloid precursors (CMPs) expressed Flt3, but the precursor activity for both DCs and p-preDCs was within this minor Flt3+ CMP fraction. The granulocyte and macrophage precursors and pro-B cells did not express Flt3 and had no DC or p-preDC precursor activity. These findings demonstrate that the early precursors for all DC subtypes are within the BM Flt3+ precursor populations, regardless of their lymphoid or myeloid lineage orientation.
Figures
Similar articles
-
Development of plasmacytoid and conventional dendritic cell subtypes from single precursor cells derived in vitro and in vivo.Nat Immunol. 2007 Nov;8(11):1217-26. doi: 10.1038/ni1522. Epub 2007 Oct 7. Nat Immunol. 2007. PMID: 17922015
-
Development of dendritic cell system.Cell Mol Immunol. 2004 Apr;1(2):112-8. Cell Mol Immunol. 2004. PMID: 16212897 Review.
-
Flt3-ligand-mobilized peripheral blood, but not Flt3-ligand-expanded bone marrow, facilitating cells promote establishment of chimerism and tolerance.Stem Cells. 2006 Apr;24(4):936-48. doi: 10.1634/stemcells.2005-0395. Stem Cells. 2006. PMID: 16644924
-
Effect of vascular endothelial growth factor and FLT3 ligand on dendritic cell generation in vivo.J Immunol. 1999 Sep 15;163(6):3260-8. J Immunol. 1999. PMID: 10477595
-
Flt3 in regulation of type I interferon-producing cell and dendritic cell development.Ann N Y Acad Sci. 2007 Jun;1106:253-61. doi: 10.1196/annals.1392.015. Epub 2007 Mar 14. Ann N Y Acad Sci. 2007. PMID: 17360795 Review.
Cited by
-
Toll-like receptor 9 trafficking and signaling for type I interferons requires PIKfyve activity.Int Immunol. 2015 Sep;27(9):435-45. doi: 10.1093/intimm/dxv021. Epub 2015 Apr 29. Int Immunol. 2015. PMID: 25925170 Free PMC article.
-
C/EBPα is required for development of dendritic cell progenitors.Blood. 2013 May 16;121(20):4073-81. doi: 10.1182/blood-2012-10-463448. Epub 2013 Apr 1. Blood. 2013. PMID: 23547051 Free PMC article.
-
Spleen as a site for hematopoiesis of a distinct antigen presenting cell type.Stem Cells Int. 2011;2011:954275. doi: 10.4061/2011/954275. Epub 2011 Nov 15. Stem Cells Int. 2011. PMID: 22190965 Free PMC article.
-
Generation of functionally distinct B lymphocytes from common myeloid progenitors.Clin Exp Immunol. 2007 Nov;150(2):349-57. doi: 10.1111/j.1365-2249.2007.03493.x. Epub 2007 Sep 5. Clin Exp Immunol. 2007. PMID: 17822442 Free PMC article.
-
Calcium and calcineurin-NFAT signaling regulate granulocyte-monocyte progenitor cell cycle via Flt3-L.Stem Cells. 2014 Dec;32(12):3232-44. doi: 10.1002/stem.1813. Stem Cells. 2014. PMID: 25100642 Free PMC article.
References
-
- Lyman, S.D., L. James, T. Vanden Bos, P. de Vries, K. Brasel, B. Gliniak, L.T. Hollingsworth, K.S. Picha, H.J. McKenna, R.R. Splett, et al. 1993. Molecular cloning of a ligand for the flt3/flk-2 tyrosine kinase receptor: a proliferative factor for primitive hematopoietic cells. Cell. 75:1157–1167. - PubMed
-
- Hannum, C., J. Culpepper, D. Campbell, T. McClanahan, S. Zurawski, J.F. Bazan, R. Kastelein, S. Hudak, J. Wagner, J. Mattson, et al. 1994. Ligand for FLT3/FLK2 receptor tyrosine kinase regulates growth of haematopoietic stem cells and is encoded by variant RNAs. Nature. 368:643–648. - PubMed
-
- Gilliland, D.G., and J.D. Griffin. 2002. The roles of FLT3 in hematopoiesis and leukemia. Blood. 100:1532–1542. - PubMed
-
- Rasko, J.E., D. Metcalf, M.T. Rossner, C.G. Begley, and N.A. Nicola. 1995. The flt3/flk-2 ligand: receptor distribution and action on murine haemopoietic cell survival and proliferation. Leukemia. 9:2058–2066. - PubMed
-
- Brasel, K., S. Escobar, R. Anderberg, P. de Vries, H.J. Gruss, and S.D. Lyman. 1995. Expression of the flt3 receptor and its ligand on hematopoietic cells. Leukemia. 9:1212–1218. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

